Thermochemistry
Thermochemistry: Overview
This topic covers concepts such as thermochemistry, enthalpy of reaction, enthalpy of formation, standard enthalpy of formation and standard enthalpy of combustion.
Important Questions on Thermochemistry
The following two reactions are known:
The value of for the following reaction
From the data of following bond energies:
Calculate the enthalpy of the following reaction in .
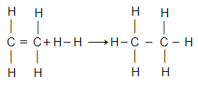
Given that bond energies of respectively and for , bond enthalpy of is:
Consider the following reactions
(i)
(ii)
(iii)
(iv)
Enthalpy of formation of
The enthalpy of hydrogenation of cyclohexene is If resonance energy of benzene is its enthalpy of hydrogenation of benzene would be
For which one of the following equations is equal to for the product?
Enthalpy of is negative. If enthalpy of combustion of and are x and y respectively, then which relation is correct:
The values of heat of formation of are –298.2 kJ and –98.2 kJ. The enthalpy change of the reaction
will be
Bond dissociation enthalpy of respectively. The enthalpy of formation of HCl is:
What is enthalpy of hydration ?
The number of endothermic process/es from the following is _______.
A.
B.
C.
D.
E. Dissolution of ammonium chloride in water
and are diatomic molecules. If the bond enthalpies of and are in the ratio then the bond enthalpy of is (Nearest integer)
Solid fuel used in rocket is a mixture of and (in ratio ). The heat evolved per gram of the mixture is _____ (Nearest integer)
Given
Molar mass of and are and respectively
Consider the following data
Heat of combustion of
Heat of combustion of
Heat of combustion of
The heat of formation of is _____ (Nearest integer).
Given
(A)
(B)
The for the reaction is
The values of enthalpy of formation for the compounds respectively. Find out which one is better fuel between
Determine the enthalpy change for the given reaction:
Bond energies are given as below:
For the reaction,
If the what is ?
Find the for the reaction below, given the following reactions and subsequent values:
Heat of combustion of naphthalene is at constant volume and . What will be the heat of combustion of the reaction at constant pressure and ?
