Batteries
Batteries: Overview
This topic covers concepts, such as Electrical Batteries, Dry Cell, Lead Storage Battery, Discharging of Lead Storage Battery, Charging of Lead Storage Battery, Nickel-Cadmium Storage Cell, Hydrogen-Oxygen Fuel Cell, etc.
Important Questions on Batteries
The type of cell in a lead storage battery is:
Select the incorrect statement while a lead storage battery is discharging.
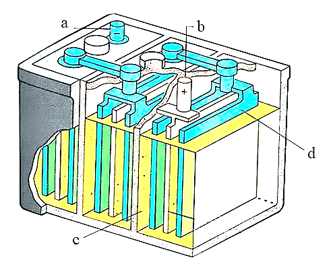
Which of the following is correct labelling of lead storage battery:
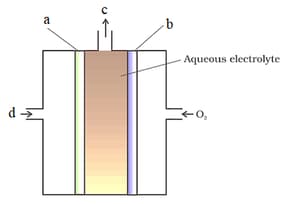
In hydrogen-oxygen fuel cell, the carbon rods are immersed in hot aqueous solution of:
Which of the following are correctly label for fuel cell
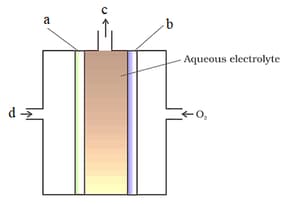
Which of the following is correct labelling of fuel cell:
The anode reaction which is taking place in nickel- cadmium battery can be represented by which of the following equation?
During the discharging of a lead storage battery the reaction occurring at anode is represented by:-
In the lead-acid battery during charging, the cathode reaction is
In fuel cell the reaction occurring at cathode is
Which of the following reaction occurs at anode during the recharging of lead storage battery ?
Which of the following reaction occurs at cathode in fuel cell ?
Which of the following will be formed when lead storage battery is charged?
While charging the lead storage battery ______.
A lead storage battery consists of a lead anode and a grid of lead packed with lead dioxide as the cathode. The electrolyte taken is 39% by mass having a density of The battery holds 3.5 L of the acid. During the discharge of the battery, the density falls from to which is 20% by mass.
Moles of sulphuric acid lost during discharge is:
Which represents the positive electrode of a nickel-cadmium battery?
