Electrolytic Cells and Electrolysis
Electrolytic Cells and Electrolysis: Overview
In this topic, we will learn about electrolytic cells and electrolysis. Here we will learn that an electrolytic cell drives a spontaneous redox reaction through electrical energy. Electrolysis, however, is a technique that uses direct current.
Important Questions on Electrolytic Cells and Electrolysis
What amount of silver will be deposited by passing electricity through two solutions containing and .
The image below shows electrolysis of an electrolyte using a DC voltage source.
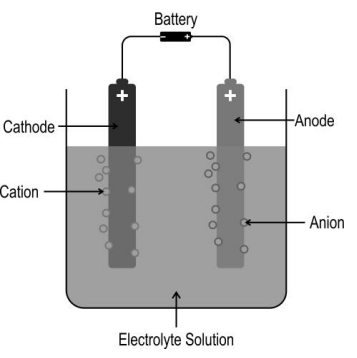
Based on this, Which of the following statements is/are correct?
(i) The solution remains electrically neutral during electrolysis.
(ii) Electrons flow from the current source towards the solution at one electrode, and an equal number of electrons flow away from the solution at the other electrode.
(iii) The number of positive ions moving towards one electrode is always equal to the number of negative ions moving towards the other electrode.
Write a anodic reaction involved in the electrolysis of brine solution using mercury electrode.
Write the reactions that occur on anode and cathode during electrolysis of dilute sulphuric acid.
The product obtained at cathode during electrolysis of dilute sulphuric acid is:
The product obtained at cathode during electrolysis of dilute sulphuric acid is hydrogen gas.
The product obtained at anode during electrolysis of dilute sulphuric acid is hydrogen gas.
Electrolysis of solution gives _____ at anode. (chlorine / oxygen)
A sample of , dissolved in a molten fluoride bath, is electrolysed using a current of What is the rate of production of in ?
Give your answer by multiplying with 1000 and rounding off to three significant figures.
A solution containing and cyanide complexes was electrolysed and a deposit of was obtained. The deposit contained and . No other element was released. Calculate the number of coulombs passed through the solution. (At. wt. )
Report the answer by rounding off to three significant figures
If of of solution is electrolysed by a current of for ,
The number of grams of the product deposited at the cathode is , Report the value of significant figures.
Lake Cayuga has a volume of water estimated to be . A power station not so far above Cayuga's waters produces electricity at the rate of at an appropriate voltage. How long(in years) would it take to electrolyse the lake?
If the answer is
How long (in sec) should a current of be passed through of a solution in order to make its , assuming that no volume changes?
Round-off the answer to the nearest integer.
One hundred millilitres of copper sulphate solution is electrolysed for minutes by passing a current of . Calculate the amount of copper sulphate in grams in the solution.
Round off answer to the nearest integer value.
Electric current is passed through two cells in series. Cell contains an aqueous solution of and platinum electrodes. The cell contains an aqueous solution of and electrodes. The current is passed till of oxygen is liberated at the anode of cell
Calculate the mass of silver deposited(in grams) at the cathodes (Round off answer to the nearest integer value).
For the electrolytic production of from as per reaction:
How many faradays of electricity would be required to produce of ?
of a solution is electrolysed for at a current of . If is produced at one electrode and at the other, what will be the of the final solution? For ,
Report your answer by multiplying the PH value obtained in to 10 and rounding off the value to two significant figures.
How many hours are required for a current of to decompose electrolytically of water?
Express your answer by rounding off the value to two significant figures.
During the electrolysis of aqueous solution of by using platinum electrodes _____ will deposit at cathode.(Copper/Oxygen)
During electrolysis of a solution of , coulombs of charge is passed through the solution. The mass of silver deposited on the cathode is _____ .
