Reference Electrodes
Reference Electrodes: Overview
This topic covers concepts, such as, Reference Electrodes, Standard Hydrogen Electrode & Calomel Electrode etc.
Important Questions on Reference Electrodes
A standard hydrogen electrode has zero electrode potential because
Standard hydrogen electrode (SHE) is a
The electrode potential of is fixed as
The standard hydrogen electrode has zero electrode potential because
During the course of reaction, a half cell is connected with SHE, pH of SHE is increased. The connected half cell behaves as:
What would be of hydrogen electrode having ?
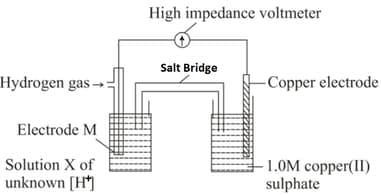
What could be the best material for electrode , if a student set up the following apparatus to determine the hydrogen ion concentration of solution X.
The cell is .
Consider the cell
Given:
Calculate the for
In which of the following electrochemical cell overall cell reaction is :
Consider the cell: . The cell potential:
A standard hydrogen electrode has zero electrode potential because:
Which one of the following does not hold good for S.H.E (Standard Hydrogen Electrode) ?
The reduction potential of hydrogen half-cell will be negative if
