Relationship between Equilibrium Constant (K), Reaction Quotient (Q) and Gibbs Energy (G)
Relationship between Equilibrium Constant (K), Reaction Quotient (Q) and Gibbs Energy (G): Overview
This topic covers the concept Relationship between Equilibrium Constant (K), Reaction Quotient (Q) and Gibbs Free Energy Change (ΔG).
Important Questions on Relationship between Equilibrium Constant (K), Reaction Quotient (Q) and Gibbs Energy (G)
The Haber’s process for the formation of at is . Which of the following is the correct statement?
Consider the curve between and . Which plot will be correct for exothermic reaction?
The ionisation constant of in water is at . The rate constant for the reaction of and to form and at is . If dissociation constant of water at is . If of water at is then the heat of neutralisation is:
The change in standard Gibbs energy for a reaction at equilibrium, $eg . \mathrm{PCl}_{5}(g) \rightleftharpoons \mathrm{PCl}_{3}(g)+\mathrm{Cl}_{2}(g),$ on addition of an inert gas at constant pressure and then at constant volume respectively are :
moles of taken in a one litre flask dissociates to extent to reach equilibrium. What is of the reaction ?
the equilibrium pressure of at and are and bar respectively. Then :
Consider the reaction :
Then the composition of the equilibrium mixture at is
The equilibrium constant for a reaction is 10. will be
What are the values of and the equilibrium constant for the formation of from and at K. Given
where
Calculate for the reaction
at 298 K for the reaction is is
Which of the following statements is correct for a reversible process in a state of equilibrium?
A schematic plot of versus for a reaction is shown below:
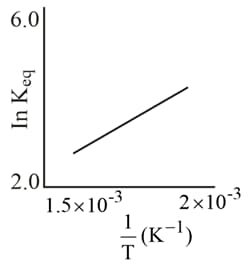
The reaction must be:
The value of for a reaction is?
(Given, and
The standard entropies of and are and , respectively. For the reaction , to be at the equilibrium, the temperature should be
Which is/are correct ?
For the equilibrium at ; and . moles of and moles of are taken initially in a one-litre container, then, which statements are correct?
The correct statement regarding a reversible system is (are):
The equilibrium constant of the following reaction in equilibrium at
is 10
Which of the following statements are correct?
Which of the following statements is true?
For a given reaction, at .
What is the value of in ?
