Theory of Radioactive Disintegration
Theory of Radioactive Disintegration: Overview
This topic covers concepts, such as, Activity of a Radioactive Substance, Units of Radioactivity, Magic Numbers & Liquid Drop Model etc.
Important Questions on Theory of Radioactive Disintegration
The half-life for decay of radioactive is 5730 years. An archaeological artifact containing wood has only 80% of the activity as found in living trees. The age of the artifact would be:
[Given: log 1.25 = 0.0969]
The half life of a radioactive isotope is three hours. If the initial mass of the isotope were 256 g, the mass of it remaining undecayed after 18 hours would be
particle is emitted in a radio active reaction when
Two radioactive processes are given below
The values for these two processes are respectively, are given by
[Here denotes the mass of an element of , denotes the mass of electron and denotes the amount energy liberated when 1 u of mass converts to energy]
Which of the block group will change its group on emitting particle (alpha particle)
Correct answer is _____________.
A rock is found to contain helium at STP and of uranium ( of is and each is converted to ).
Thus, age of rock is
THE THORIUM SERIES:
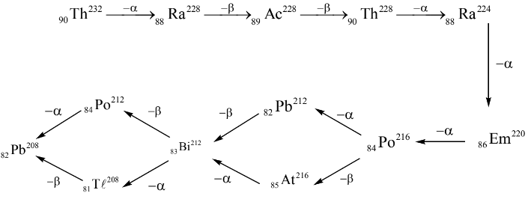
Find the number of correct statements about the given series.
1. It is one of the natural series found on earth.
2. The number of alpha particles obtained on disintegration of one atom to get the stable product is .
3. The number of beta particles obtained on disintegration of one atom to get the stable product is .
4. and are isodiaphers.
5. The nuclear isomers of gives on beta decay and on alpha decay.
If is atomic mass, is a mass number, What is meaning of ?
Define magic numbers of nucleus of an atom.
The half-life of is What mass of this nuclide (in mg) has an activity of
(Given )
Report your answer up to two places of decimal.
The disintegration rate for a sample containing as the only radioactive nuclide, is found to be atoms/minute. of is years. Find the number of atoms of in the sample. How long(in years) must this radioactive sample be maintained before the rate falls to disintegration per minute?
Calculate the weight (in ) of atoms which will give disintegration per second ().
If of a radioactive substance has of the same substance will have a equal to
The rate of the process:
Radioactive disintegration rate is affected by
Half-life of a radioactive sample is years. What fraction of this sample will remain undecayed after years?
Which of the following statements are correct?
Which of the following statements are correct?
