Theory of Radioactive Disintegration
Theory of Radioactive Disintegration: Overview
This topic covers concepts, such as, Activity of a Radioactive Substance, Units of Radioactivity, Magic Numbers & Liquid Drop Model etc.
Important Questions on Theory of Radioactive Disintegration
The half life of a radioactive isotope is three hours. If the initial mass of the isotope were 256 g, the mass of it remaining undecayed after 18 hours would be
If is atomic mass, is a mass number, What is meaning of ?
Define magic numbers of nucleus of an atom.
The half-life of is What mass of this nuclide (in mg) has an activity of
(Given )
Report your answer up to two places of decimal.
If of a radioactive substance has of the same substance will have a equal to
The rate of the process:
Radioactive disintegration rate is affected by
Half-life of a radioactive sample is years. What fraction of this sample will remain undecayed after years?
One becquerel of radioactivity is equal to
The nuclide lying below the stability belt in graph does not disintegrate by
A radioactive nuclide generally disintegrates by emission when its ratio is
The number of stable isotopes is the least when the number of neutrons and protons in the isotopes are, respectively,
The binding energy per nucleon of is and that of is . The energy in required to remove a neutron from is
Both technetium- and thallium- are used to image heart muscle in patients who may have heart problems. The half-lives are and respectively. What percent of radioactivity would remain for each of the isotopes after days?
One drug has radioactivity 80 dpm and after 20 minutes its activity is 40 dpm. How many number of atoms present initially ?
Calculate the age of rock if a radioactive isotope X with half-life of years decay to Y which is stable. A sample of rock from the moon was found to contain both the element X and Y in the mole ratio 1 : 7.
Calculate the mass left undecayed after 6 hour if the half-life of a radioisotope is hours and its initial mass is .
Calculate the number of atoms present intially, where A drug has radioactivity 80 dpm and later its activity is increased to 40 dpm after 20 minutes.
Among the following, what is the correct order for the decay constant of three radioactive species A, B and C?
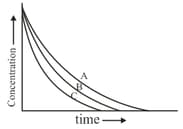
If two radioactive sample with equal number of nuclei have decay constant and , the ratio of respective number of nuclei after a time is
