Oxidation Number
Oxidation Number: Overview
This topic covers concepts such as Oxidation Number, Stock Notation for Oxidation Number, Rules for Calculating Oxidation Number, Oxidation Number in Native State, Oxidation Number of H-atom in compounds, Oxidation Number of O-atom in Compounds, etc.
Important Questions on Oxidation Number
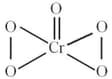
has a structure as shown below:
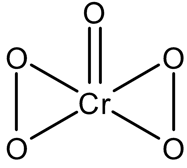
The oxidation number of chromium in the above compound is
has structure as shown

Which of the following oxidation states is/are shown by hydrogen?
The oxidation state of chromium in the final product formed by the reaction between and acidified potassium dichromate solution is
mole of (atomic weight is ) is oxidized to . Calculate the equivalent weight of ferrous ion
One mole of hydrazine loses 10 moles of electrons in a reaction to form a new compound Assuming that all the nitrogen atoms in hydrazine appear in the new compound, what is the oxidation state of nitrogen in ? (Note There is no change in the oxidation state of hydrogen in the reaction)
Which of the following oxidation states is/are shown by hydrogen?
What is the oxidation number of copper in brass?
The oxidation state of nitrogen in dinitrogen trioxide is:
The heating of produces another chromium compound along with gas. The change of the oxidation state of in the reaction is
The formal oxidation numbers of and in the ions and , respectively are
How many of the following species have formal oxidation state of or as
The species with an atom in oxidation state is
Sulphur and rest of the elements of group 16 are less electronegative than oxygen. Therefore, their atoms cannot take up electrons easily. They can acquire ns2np6 configuration by sharing two electrons with the atoms of other elements and thus, exhibit +2 oxidation state in their compounds. In addition to this, their atoms have vacant d-orbitals in their valence shell to which electrons can be promoted from the p and s-orbitals of the same shell. As a result, they can show +4 and +6 oxidation states.
Like sulphur, oxygen does not show +4 and +6 oxidation states. The reason is :
Sulphur and rest of the elements of group 16 are less electronegative than oxygen. Therefore, their atoms cannot take up electrons easily. They can acquire ns2np6 configuration by sharing two electrons with the atoms of other elements and thus, exhibit +2 oxidation state in their compounds. In addition to this, their atoms have vacant d-orbitals in their valence shell to which electrons can be promoted from the p and s-orbitals of the same shell. As a result, they can show +4 and +6 oxidation states.
The oxidation state of sulphur in S8, SO3 and H2S respectively are :
In the periodic table, oxidation state _____ across a period.
Which of the following elements have an oxidation number of -1.
Which oxidation state is exhibited by the alkaline earth metals?