Amorphous and Crystalline Solids
Amorphous and Crystalline Solids: Overview
This topic covers concepts such as Types of Solids on the Basis of Nature of Arrangement of Lattice Points, Crystalline Solids, Amorphous Solids and Distinction between Crystalline and Amorphous Solids.
Important Questions on Amorphous and Crystalline Solids
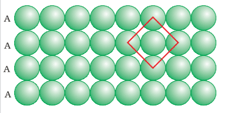
The above diagram is planar. It is a diagram of 2-D _____ close packing.
Explain the Crystalline solids have sharp melting points.
Which type of intermolecular forces are present in the crystal lattice of iodine molecules?
A solid translucent material exhibited different optical clarity when seen through, from different side. What is the reason of such observation?
Which of the following is an amorphous solid?
Which of the following is not a characteristic of a crystalline solid?
Classify the following solid as ionic, metallic, molecular network or amorphous.
Plastic
What makes a glass different from a solid such as quartz? Under what conditions quartz could be converted into glass?
Refractive index of a solid is observed to have the same value along all dissections. Comment on the nature of this solid. Would it show cleavage property?
Why a glass considered a super cooled liquid?
Classify the following as amorphous or crystalline solids :
Polyurethane, naphthalene, benzoic acid, Teflon, potassium nitrate, cellophane, polyvinyl chloride, fibre glass, copper.
Define the term 'amorphous' Give some examples of amorphous solids.
Classify the following solids into different types:
Plastic
What is a glass? Distinguish between crystalline solids and amorphous solids. Give examples.
Explain crystalline solids and amorphous solids.
Give the classification of solids.
Explain the terms isomorphism, polymorphism, anisotropy and unit cell.
Which of the following is/are pseudo solids?
.
. Barium chloride dihydrate
. Rubber
. Solid cake left after the distillation of coal tar.
Quartz is a crystalline variety of