Classification of Solids
Classification of Solids: Overview
This topic covers concepts, such as, Types of Solids on the Basis of Nature of Arrangement of Lattice Points,Crystalline Solids,Isomorphism in Ionic Crystals etc.
Important Questions on Classification of Solids
Glass is an example of _______(Crystalline/amorphous) solids.
Which of the following is a crystalline solid ?
Which of the following is NOT true about the amorphous solids?
Describe different types of solids.
Which of the following statement is correct?
What are crystalline solids? Explain the types of crystalline solids.
Is benzoic acid a crystalline solid or an amorphous solid?
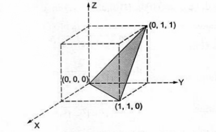
The coordinates of the three corners of a shaded face on a cubic unit cell are as shown in the figure. Determine the Miller indices of the plane.
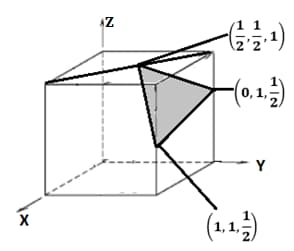
Determine the Miller indices of the shaded plane. Coordinates of the plane are shown in the figure.
How do the structures of quartz and quartz glass differ from each other?
Glass is considered as a supercooled liquid. Give reason.
Draw a diagram for anisotropic behaviour of crystalline solids.
Write two difference between crystalline solids and amorphous solids.
Account for the following:
Some of the glass objects recovered from ancient monuments look milky instead of being transparent.
What are crystalline and amorphous solids? Explain with examples.
Why urea has a sharp melting point but glass does not?
What are amorphous solids? Give their important properties and uses.
Write any two differences between amorphous solids and crystalline solids.
The property of crystalline solid is not