Close Packed Structures
Close Packed Structures: Overview
This topic covers concepts, such as, Square Close Packing in Two Dimension, Hexagonal Close Packing in Two Dimension, Effective Number of Atoms in HCP Unit Cell & Coordination Number of an HCP Unit Cell etc.
Important Questions on Close Packed Structures
The coordination number is equal to _____ in one-dimensional close packing in crystalline solid.
Describe close packing in one dimension in crystalline solids.
How many unit cells are present in a cube shaped ideal crystal of of mass
A two dimensional solid is made by alternating circles with radius and such that the sides of the circles touch. The packing fraction is defined as the ratio of the area under the circles to the area under the rectangle with sides of length and
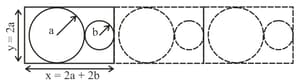
The ratio for which the packing fraction is minimized is closest to:
Atoms of an element form hexagonal closed packed lattice and atoms of element occupy all the tetrahedral voids. The formula of the compound is
The space occupied by arrangement is approximately
The number of hexagonal faces that are present in a truncated octahedron is
In a crystalline solid atoms occupy hcp and th of tetrahedral voids are occupied by atoms. Then empirical formula of crystalline solid is:
In one dimensional close packed arrangement, the coordination number is _____ .
In one dimensional close packed arrangement, the coordination number is .
Give the coordination number in close packing of lattice points in one dimension ?
The number of atoms present in a hexagonal close packed unit cell is:
A compound of formula has the lattice. Which atom forms the lattice and what fraction of tetrahedral voids are occupied by the other atoms
In the hexagonal closest packed structure of a metallic lattice, number of the nearest neighbours of a metallic atom is
The arrangement of first two layers is one above the other. In and , the arrangements are:
Which one of the following methods of ordering closed packed sheets of equal sized spheres does not generate close-packed lattice?
Atom '' is crystallised in HCP crystal structure and of the tetrahedral voids are occupied by . What is the formula for the compound?
What is the coordination number of an atom in its own layer in hexagonal closed pack arrangement?
What is the coordination number of an unit cell?
