Imperfection in Solids
Imperfection in Solids: Overview
This topic covers concepts, such as, Imperfections in Crystalline Solids, Point Defects and Line Defects, Metal Excess Defects Due to Cations in Interstitial Spaces & Metal Deficiency Defects Due to Cationic Vacancies etc.
Important Questions on Imperfection in Solids
Which of the following ionic compound will show Frenkel defect?
Which of the following point defect of crystals decreases the density of a solid?
Given below are two statements: one is labelled as Assertion and the other is labelled as Reason .
Assertion : In a particular point defect, an ionic solid is electrically neutral, even if few of its cations are missing from its unit cells.
Reason : In an ionic solid, Frenkel defect arises due to dislocation of cation from its lattice site to interstitial site, maintaining overall electrical neutrality.
In the light of the above statements, choose the most appropriate answer from the options given below:
Match the following:
| Column I (Defect) | Column II (Effect) |
| (A) Schottky defect | (p) Crystal becomes coloured |
| (B) Doping silicon with aluminium | (q) n-type semiconductor is formed |
| (C) Doping silicon with arsenic | (r) p-type semiconductor is formed |
| (D) Heating crystal in presence of sodium vapour | (s) Density of the crystal decreases |
No crystal is found to be prefect at room temperature. The defects present in the crystals can be stoichiometric or non-stoichiometric. Due to nonstoichiometric defects, the formula of the ionic compound is different from the ideal formula. For example, the ideal formula of ferrous oxide should be but actually in one sample, it was found to be . This is because the crystal may have some ferric ions in place of ferrous ions. These defects change the properties of the crystals. In some cases, defects are introduced to have crystals of desired properties as required in the field of electronics. Doping of elements of Group 14 with those of Group 13 or 15 is most common. In ionic compounds, usually impurities are introduced in which the cation has higher valency than the cation of the parent crystal, e.g., into .
Which one of the following defects does not affect the density of the crystal ?
No crystal is found to be prefect at room temperature. The defects present in the crystals can be stoichiometric or non-stoichiometric. Due to nonstoichiometric defects, the formula of the ionic compound is different from the ideal formula. For example, the ideal formula of ferrous oxide should be but actually in one sample, it was found to be . This is because the crystal may have some ferric ions in place of ferrous ions. These defects change the properties of the crystals. In some cases, defects are introduced to have crystals of desired properties as required in the field of electronics. Doping of elements of Group 14 with those of Group 13 or 15 is most common. In ionic compounds, usually impurities are introduced in which the cation has higher valency than the cation of the parent crystal, e.g., into .
was doped with . The concentration of cation vacancies is
For the number of Schottky pairs per at room temperature are
Identify the incorrect statement regarding crystals having Frenkel defect:
Non-stoichiometric cuprous oxide, Cu2O can be prepared in laboratory. In this oxide, copper to oxygen ratio is slightly less than 2 : 1. This substance is a?
Which is/are incorrect for Schottky defect in rock salt type crystals:
Which one of the following has both Schottky and Frenkel defects?
The composition of a sample of Wustite is . The percentage of ions present in the sample is about
is crystallized from molten containing a little .The solid obtained will have
Experimentally it was found that a metal oxide has formula . Metal , is present as and in its oxide. Percentage of the metal which exists as would be :-
Which one of the following compounds shows both, Frenkel as well as Schottky defects?
What type of crystal defect is indicated in the diagram below?
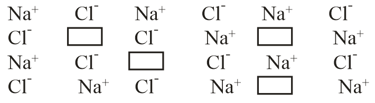
The composition of a sample of wurtzite is what of Iron present in the form of ?
