Voids
Voids: Overview
This topic covers concepts, such as Octahedral and Tetrahedral Voids, Cubical Voids, Voids and their Types, Triangular Planar Voids, and Number and Locations of Tetrahedral and Octahedral Voids in fcc.
Important Questions on Voids
Interstitial sites that are formed when three closed packed spheres of one layer is put on three close packed spheres of the second layer, their positions being inverted with respect to each other.
Number of moles of tetrahedral voids present in type structure having moles of atoms is
The empty space between the shaded balls and hollow balls as shown in the diagram is called
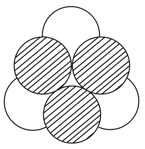
The number of octahedral sites in a cubical close pack array of spheres is
A unit cell is obtained by closed packing layers of atoms in ....... pattern. The total number of tetrahedral and octahedral voids in the unit cell are respectively
The type of void present at the centre of the ccp unit cell is:
Gold crystallises in ccp structure. The total number of voids present in of gold will be
'' has fcc arrangement, '' is present in of tetrahedral voids. The formula of the compound will be
Total number of octahedral voids present per unit cell of unit cell is:
Octahedral void at edge centre in arrangement is equally distributed amongst:
The number of tetrahedral voids present on each body diagonal in ccp unit cell is
A compound is formed by two elements and . The element forms ccp and atom occupies of the tetrahedral voids. The formula of the compound is
| Column I (Type of crystal) | Column II (Location of cations/anions) |
| (A) | (p) Cations-fcc, Anions-all tetrahedral voids |
| (B) | (q) Anions-fcc, Cations-all tetrahedral voids |
| (C) | (r) Anions-fcc, Cations-all octahedral voids |
| (D) | (s) Anions-fcc, Cations-alternate tetrahedral voids |
A molecule have radius ratio of is more than 0.732. Which of the following void is presented in the molecule.
A molecule have radius ratio of is 0.200 . Which of the following void is presented in the molecule.
Right option for the number of tetrahedral and octahedral voids in hexagonal primitive unit cell are:
In a compound, atoms of element form cubic close packing lattice and those of element occupy of tetrahedral voids. The formula of the compound will be
The total number of tetrahedral voids in the face centred unit cell is
In a carbon allotrope having lattice, two atoms are at and coordinates. What is the ratio of bond length distance to the edge length of the unit cell?
