Vapour Pressure of Liquid Solutions
Vapour Pressure of Liquid Solutions: Overview
This topic covers concepts, such as, Vapour Pressure, Variation of Vapour Pressure with Temperature, Partial Vapour Pressure, Saturated Vapour Pressure, Boiling Point, Standard Boiling Point & Freezing Point etc.
Important Questions on Vapour Pressure of Liquid Solutions
Assertion: When is added to water a depression in the freezing point is observed.
Reason: The lowering of the vapour pressure of a solution causes depression in the freezing point.
Which of the following solutions does not show positive deviation?
A person living in Shimla observed that cooking food without using pressure cooker takes more time. The reason is that at high altitude
The plot of total vapour pressure as a function of mole fraction of the components of an ideal solution formed by mixing liquids and is
What is the correct increasing order of vapour pressure of water, acetone and ether at a temperature of ? Given that among these compounds, water has maximum boiling point and ether has minimum boiling point.
The vapour pressure of water at is of .
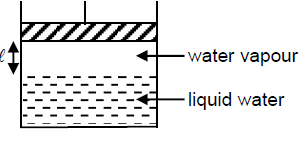
Which of the following will ensure that pressure of vapour above liquid will not change?
At and one atm, 'a' litre of decomposes to as: To what extent has the decomposition proceeded when the original volume is 25% less than that of existing volume?
[Report your answer in two significant figures.]
The vapour pressure of water at is . The following solutions are prepared at
of urea (molecular weight ) is dissolved in of water.
of glucose is dissolved in of water.
of (molecular weight ) is dissolved in of water.
Identify the correct order in which the vapour pressure of solutions increases
Vapour pressure of a liquid increases with
A person living at high altitude region observed that cooking food without using pressure cooker takes more time. The reason for this observation is that
Which of the following statements is correct?
The pair of boiling point and compound are given as,
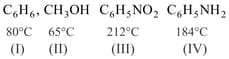
Which will show lowest vapour pressure at room temperature?
At a constant temperature, which of the following aqueous solutions will have the maximum vapour pressure?
Statement - : Only temperature can change the vapour pressure of a pure liquid.
Statement - : Equilibrium constant does not change unless temperature is changed.
A liquid is kept in a closed vessel. If a glass plate of negligible mass with small hole is kept on top of the liquid surface, then vapour pressure of the liquid in the vessel is:
Which one of the statements given below-concerning properties of solutions, describes a colligative effect?
The vapour pressure of a liquid in a closed container depends upon
On a humid day in summer, the mole fraction of gaseous a (water vapour) in the air at can be as high as 0.0287. Assuming a total pressure of 0.977 atm. What is the partial pressure of dry air?
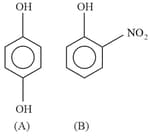
Out of the compounds below, the vapour pressure of (B) at a particular temperature is:
Using the following information determine the boiling point of a mixture contains 1560 g benzene and 1125 g chlorobenzene, when the external pressure is 1000 torr. Assume the solution is ideal.
Given, Molar mass of benzene = 78
Molar mass of chlorobenzene = 112.5
| Temperature (0oC) |
Vapour pressure of benzene (torr) |
Vapour pressure of chlorobenznee (torr) |
|---|---|---|
| 80 | 750 | 120 |
| 90 | 1000 | 200 |
| 100 | 1350 | 300 |
| 110 | 1800 | 400 |
| 120 | 2200 | 540 |
