Vapour Pressure
Vapour Pressure: Overview
This topic covers concepts, such as, Vapour Pressure, Boiling Point & Molar Heat of Vapourisation etc.
Important Questions on Vapour Pressure
Why molar heat of vaporisation of water is greater than hexane?
Molar heat of vaporisation of water is greater than hexane.
What do you mean by molar heat of vaporization?
The plot of total vapour pressure as a function of mole fraction of the components of an ideal solution formed by mixing liquids and is
The vapour pressure of water at is of .
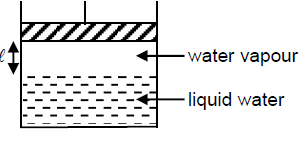
Which of the following will ensure that pressure of vapour above liquid will not change?
Which of the following statements is correct?
The pair of boiling point and compound are given as,
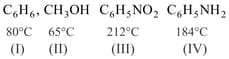
Which will show lowest vapour pressure at room temperature?
At a constant temperature, which of the following aqueous solutions will have the maximum vapour pressure?
A liquid is kept in a closed vessel. If a glass plate of negligible mass with small hole is kept on top of the liquid surface, then vapour pressure of the liquid in the vessel is:
Which one of the statements given below-concerning properties of solutions, describes a colligative effect?
The vapour pressure of a liquid in a closed container depends upon
On a humid day in summer, the mole fraction of gaseous a (water vapour) in the air at can be as high as 0.0287. Assuming a total pressure of 0.977 atm. What is the partial pressure of dry air?
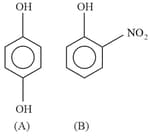
Out of the compounds below, the vapour pressure of (B) at a particular temperature is:
Using the following information determine the boiling point of a mixture contains 1560 g benzene and 1125 g chlorobenzene, when the external pressure is 1000 torr. Assume the solution is ideal.
Given, Molar mass of benzene = 78
Molar mass of chlorobenzene = 112.5
| Temperature (0oC) |
Vapour pressure of benzene (torr) |
Vapour pressure of chlorobenznee (torr) |
|---|---|---|
| 80 | 750 | 120 |
| 90 | 1000 | 200 |
| 100 | 1350 | 300 |
| 110 | 1800 | 400 |
| 120 | 2200 | 540 |
The aqueous solution that has the lowest vapour pressure at a given temperature is:
A substance will be deliquescent if its vapour pressure :
Which of the following liquid will exhibit highest vapour pressure ?
