Ideal Behavior of Gases
Ideal Behavior of Gases: Overview
This topic covers concepts, such as, Ideal Gas etc.
Important Questions on Ideal Behavior of Gases
Internal energy of mol of hydrogen of temperature is equal to the internal energy of mol of helium at temperature . The value of is
One mole of an ideal gas present in a closed vessel, is compressed to one third of its original volume under constant temperature then increase in pressure of gas is -
The diameter of a balloon filled by moles of He gas is . If moles of He gas effuses out in the night, then what would be the diameter of balloon in the next morning? Assume constant pressure and temperature for the gas.
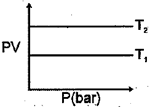
The product of is plotted against at two temperatures and and the result is shown in the figure. What is correct about and
An open vessel at is heated until two-fifth of the air (assumed as an ideal gas) in it has escaped from the vessel. Assuming that the volume of the vessel remains constant, the temperature at which the vessel has been heated is:
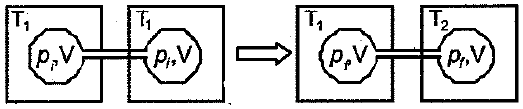
Two closed bulbs of the equal volume containing an ideal gas initially at pressure and temperature are connected through a narrow tube of negligible volume as shown in the figure below. The temperature of one of the bulbs is then raised to . The final pressure is:
If size of a hydrogen molecule is assumed to be then calculate the ratio of molar volume to atomic volume for of hydrogen gas.
Which of the following is true for the maximum deviation of a gas from its ideal behavior?
Which of the following relation is correct according to Kinetic Theory of gases? (Symbols have their usual meanings.)
Considering the following graph for an ideal solution, find the correct statement(s).
The density of a gas is three times that of a gas . If the molecular mass of is then the molecular mass of gas is
To an evacuated empty vessel which has a movable piston under external pressure of atm, of and of an unknown gas (vapour pressure atm at ) are introduced. Assuming ideal gas behavior, the total volume of the gases at is------
A cylinder of compressed gas that bears no label is supposed to contain either ethane or ethene. Combustion of the sample shows that of the gas require of oxygen for complete combustion. This shows that the gas is
The drain cleaner, drainex contains small bits of aluminium which react with caustic soda to produce dihydrogen. What volume of dihydrogen in at and one bar will be released when of aluminium reacts?
(Take )
Which of the following graphs is inconsistent with ideal gas behaviour ?
Match the conditions of matrix from List-I with List-II, for ideal gas.
| List-I | List-II | ||
|---|---|---|---|
| P. | versus P at constant T and n | 1. |  |
| Q. | V versus at constant P and n | 2. | 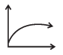 |
| R. | PT versus T2 at constant V | 3. | 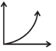 |
| S. | V versus at constant T and n | 4. | 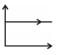 |
| 5. | 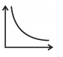 |
आदर्श गैस के लिए सूची-I तथा सूची-II से मैट्रिक्स स्थितियों को सुमेलित कीजिए
.
| सूची -I | सूची -II | ||
|---|---|---|---|
| P. | नियत T व n पर व P के मध्य ग्राफ | 1. |  |
| Q. | नियत P व n पर V व के मध्य ग्राफ | 2. |  |
| R. | नियत V पर PT व T2 के मध्य ग्राफ | 3. |  |
| S. | नियत T व n पर V व के मध्य ग्राफ | 4. |  |
| 5. |  |
Select the correct combination
सही संयोजन का चयन कीजिए
An ideal gas has pressure , volume and absolute temperature . If is the mass of each molecule and is the Boltzmann constant then density of the gas is
Match the following
| List – I | List – II | ||
| Viscosity | Critical temperature | ||
| Ideal gas behaviour | Isobar | ||
| Liquefaction of gases | Compressibility factor | ||
| Charle’s law | |||
The drain cleaner, Drainex contains small bits of aluminum which react with caustic soda to produce dihydrogen. What volume of dihydrogen at and one bar will be released when aluminum reacts?
Internal energy of mol of hydrogen of temperature is equal to the internal energy of mol of helium at temperature . The ratio is
