Liquefaction of Gases
Liquefaction of Gases: Overview
This topic covers concepts, such as, Liquefaction of Gases, Andrew's Isotherms, Compressibility Factor at Critical State & Tendency of Liquefaction of a Gas etc.
Important Questions on Liquefaction of Gases
According to the Thomas Andrews, what is the critical temperature () of carbon dioxide?
The critical volume of a gas is . What will be the radius of the molecule in ?
The radius of a molecule is . Calculate the critical volume of the gas.
The critical volume of a gas is . What will be the radius of the molecule in ?
What is critical volume? Give its formula.
The critical temperature of is less than because the molecules have:
The expression of in terms of and is:
Define critical temperature of the gas. Give its expression.
From Andrew's Isotherm experiment can be calculated.
For which compound Andrew Thomson performed his experiment.
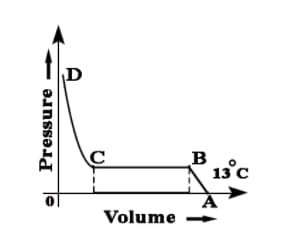
While performing experiment based on Andrew's Isotherm Explain point C and point D and line CD.
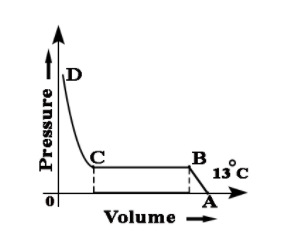
While performing experiment based on Andrew's Isotherm Explain point B and point C and line BC.
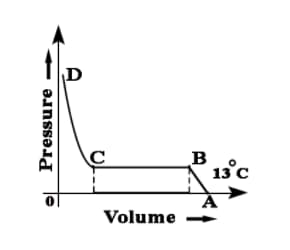
While performing an experiment for based on Andrew's Isotherm explain point A and point B and line AB.
Which of the following will show Tyndall effect?
The van der Waals constant 'a' for different gases are given below:
| Gas | () |
The gas that can be most easily liquefied is
Which one of the following gases has the highest critical temperature?
Maximum deviation from ideal gas is expected from:
If helium is allowed to expand in vacuum, it liberates heat because.
The gas which can be liquefied most easily is
The expression of in terms of and is:
