Atomic Spectra
Atomic Spectra: Overview
This topic covers concepts, such as, Hydrogen Emission Spectrum, Explanation of Line Spectrum of Hydrogen, Line Atomic Spectrum & Spectrum etc.
Important Questions on Atomic Spectra
The frequency of oscillation of the electric field vector of a certain electromagnetic.wave is Hz. What is the frequency of oscillation of the corresponding magnetic field vector and to which part of the electromagnetic spectrum does it belong?
The spectrum of He is expected to be similar to that
In the Balmer series of atomic spectra of hydrogen, there is spectral line having wavelength . Calculate the number of higher orbit from which the electron drops to generate this line.
What are some natural sources of continuous spectra?
The series of lines present in the visible region of the hydrogen spectrum is
In the Balmer series of atomic spectra of hydrogen, there is spectral line having wavelength . Calculate the number of higher orbit from which the electron drops to generate this line.
What transition in the spectrum would have the same wavelength as the first Lyman transition of hydrogen ?
When electrons from higher orbits of hydrogen atom jump to the -shell, to which series of the hydrogen spectrum will the emitted light belong?(Lyman/Balmer)
When the electron of a hydrogen atom jumps from to state, the number of spectral lines emitted is .
The series of lines present in the visible region of the hydrogen spectrum is _____ (Lyman/Balmer).
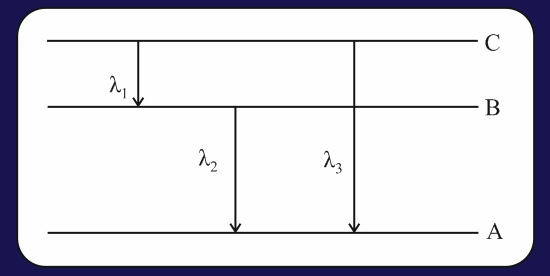
Energy levels of of a certain atom correspond to the increasing value of energy, i.e.. If
The range of wavelengths covering red colour to violet colour is called the _____ spectrum.
Emergency signals and danger signals are red because red light has high frequency than other colours.
If a player is wearing a uniform consisting of a red shirt and a white short as seen in a white light. So, their true colours are red and white respectively.
An object seen as red will reflect red colour and will absorb all other colours while the object seen as white will reflect all the colours and will not absorb any colour.
The extreme colours in the pure spectrum are blue and red.
X-rays help us to study the structure of crystals.
Choose the correct order of wavelength for below mentioned four regions of electromagnetic spectrum:
(Infrared rays, Radio waves, Microwaves, Ultraviolet rays)
The wavelength of red colour is and that of blue is . If and be the speed of red and blue colours respectively in glass. Then which of the following is correct?
