Spectrum
Spectrum: Overview
This topic covers concepts such as Spectrum, Line Atomic Spectrum, Explanation of Line Spectrum of Hydrogen, Emission Spectrum, Continuous Spectra, Hydrogen Emission Spectrum, Differences between Emission and Absorption Spectrum, etc.
Important Questions on Spectrum
The spectrum of He is expected to be similar to that
The sodium spectrum is dominated by the bright doublet known as Sodium D-lines.
Sodium light gives emission spectrum having two yellow lines.
The sodium spectrum is dominated by the bright doublet known as _____.
Sodium light gives emission spectrum having two _____.
The absorption spectrum and emission spectrum are formed due to the absorption of the energy by electrons.
The absorption spectrum comprises dark lines or gaps in the spectrum whereas the emission spectrum comprises coloured lines.
The absorption spectrum comprises _____ in the spectrum whereas the emission spectrum comprises coloured lines.
The absorption spectrum comprises dark lines or gaps in the spectrum whereas the emission spectrum comprises _____.
Differentiate absorption spectrum from emission spectrum.
Can we find the Ionisation Energy of Hydrogen in the Emission Spectrum?
Define emission spectrum for any element or compound.
What are some natural sources of continuous spectra?
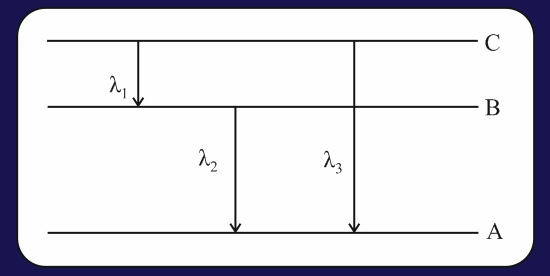
Energy levels of of a certain atom correspond to the increasing value of energy, i.e.. If
There are many types of electromagnetic radiations, which differ from one another in wavelength (or frequency).
The study of emission or absorption spectra is referred to as
When atoms are excited they emit light of certain wavelengths which correspond to different colours. This series of coloured lines is called a _____ spectra.(line/band)
Give two difference between emission and absorption spectrum.
