Towards Quantum Mechanical Model of the Atom
Towards Quantum Mechanical Model of the Atom: Overview
This topic covers concepts, such as, Quantum Mechanical Model of Atom, de-Broglie's Equation: Dual Nature of Matter, Radial Probability Density & Plot of Radial Probability Function etc.
Important Questions on Towards Quantum Mechanical Model of the Atom
A 0.66 kg ball is moving with a speed of 100 m/s. The associated wavelength will be
The measurement of the electron position if associated with an uncertainty in momentum which is equal to . The uncertainty in electron velocity is,
(mass of an electron is )
If uncertainty in position and momentum are equal, then uncertainty in velocity is :
Given : The mass of electron is , Planck constant is the uncertainty involved in the measurement of velocity within a distance of is
Set of isoelectronic species is
The position of both, an electron and a helium atom is known within . Further, the momentum of the electron is known with in .The minimum uncertainty in the measurement of the momentum of the helium atom is
The position of both, an electron and a helium atom is known within 1.0 nm. Further the momentum of the electron is known within The minimum uncertainty in the measurement of the momentum of the helium atom is
The electron was shown experimentally to have wave properties by
An electron beam with a de Broglie wavelength of is accelerated till its wavelength is halved. By what factor will kinetic energy change-
For s-orbital, vs is plotted. Select correct statement (s).
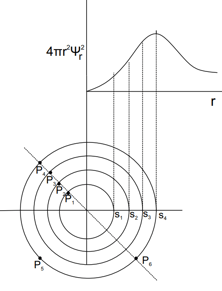
Note: S = spherical surface at given distance. P = Point (very small volume) at given distance
How many of these orbitals have maximum orbital angular probability distribution at an angle of to the axial direction?
An element undergoes a reaction as shown:
energy released
If the energy released, is used to dissociate of molecules, equally into and , where is an excited state, in which the electron travels a path length equal to four times it's de-Broglie wavelength. Determine the least amount (in moles) of that would be required.
Given: I.E. of , Bond energy of .
The de Broglie wavelength of electron of ion is If the photon emitted upon deexcitation of this ion is made to hit atom in its ground state so as to liberate electron from it, what will be the de-Broglie's wavelength of photoelectron in ?
Round off your answer to the nearest integer.
de-Broglie's wavelength of an electron having kinetic energy is . Then, what is the value of ?
The de-Broglie wavelength of an electron travelling at percent of the speed of light was found to be . Then, what is the value of ''?
The ratio of the velocity of molecules to the velocity of molecules such that they are associated with de Broglie waves of the same wavelength is
Let the mass of a particle be and its de-Broglie wavelength . Then, what will be its velocity?
If the electron is accelerated by a potential difference of , then what will be wavelength associated with the moving electron?
Suppose the kinetic energy of a particle is doubled, de-Broglie's wavelength becomes
Is the Bohr's model consistent with the Heisenberg's uncertainty principle?
