Wave Nature of Electromagnetic Radiation
Wave Nature of Electromagnetic Radiation: Overview
This topic covers concepts such as Electromagnetic Radiations, Wave Nature of Electromagnetic Radiations, Frequency of Electromagnetic Radiations, Wavelength of Electromagnetic Radiations, and Wavenumber of Electromagnetic Radiations.
Important Questions on Wave Nature of Electromagnetic Radiation
Consider the following statements :
(i) The sun is giving out energy equally in all possible directions at the rate of .
(ii) radio isotope of carbon is formed in the upper atmosphere from cosmic rays.
(iii) New element/isotope may be produced during the nuclear reaction.
(iv) When energy is absorbed, the value of nuclear reaction will be negative.
Which of the above statements are correct ?
The velocity of electromagnetic radiation is related to its wavelength and the frequency by the formula,
What is the amplitude of the electromagnetic wave in the diagram below?
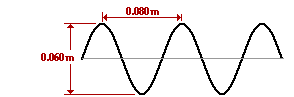
The number of wavelengths per unit length is called
What is/are correct about Hertz ?
The ratio of energy to the frequency of electromagnetic radiation is called:
An object emits two different radiation, when it absorbs certain amount of energy having of wavelength in which one radiation having What is the wavelength of other radiation?
Which value is closest to the wavelength, in meters, for a quantum of light with a frequency of per second?
[Given: ]
Which of the following is correct?
What is the correct sequence of following electromagnetic radiation arranged in the increasing order of energy?
-rays
Visible light
-rays
Select the correct answer using the codes given below:
The angular momentum of an electron in moving in an orbit is . The de-Brogile wavelength associated with electron is: [ is radius of first Bohr's orbit of -atom]
A radio can tune to any station in to band. The corresponding wavelength band is
The Vividh Bharti Station of All India Radio, Delhi, broadcasts on a frequency of 1,368 kHz (Kilohertz). Calculate the wavelength of the electromagnetic radiation emitted by transmitter. Which part of the electromagnetic spectrum does it belong to
Calculate the wavelength and energy of radiation emitted for the electronic transition from infinity to stationary state for one -atom. Given .
Choose the correct sequence of the radiation sources in increasing order of the wavelength of electromagnetic waves produced by them.
The radiation with maximum frequency is
Which of the following is never true for cathode rays?
The frequency of yellow light having wavelength is-
The wavelength of light having frequency is-
Which of the following radiations has the highest wave number ?
