Interstitial Voids
Interstitial Voids: Overview
This topic covers concepts, such as, Voids and Their Types & Octahedral and Tetrahedral Voids etc.
Important Questions on Interstitial Voids
Octahedral voids are formed when the triangular voids in second layer exactly overlap with similar voids in the first layer.
Ferric oxide crystallizes in a hexagonal close packing array of oxide ions with two out of every three octahedral holes occupied by ferric ions. Derive the formula of the ferric oxide.
If the radius of the octahedral void is and the radius of the atoms in the close packing is , derive the relationship between .
How will you distinguish between the following pairs of terms:
Tetrahedral void and octahedral void.
The number of tetrahedral sites per sphere in ccp structure is,
The number of octahedral sites per sphere in fcc structure is
The ratio of close packed atoms to octahedral holes in hexagonal close packing is
The ratio of close packed atoms to tetrahedral holes in cubic packing is
A compound is formed by two elements and . The element forms and atoms occupy of tetrahedral voids. What is the formula of the compound?
A compound forms hexagonal close packing structure. What is the total number of voids in of it? How many of these are of tetrahedral voids?
The number of tetrahedral and octahedral holes in a hexagonal primitive unit cell are:
Explain the packing and voids in ionic solids.
Distinguish between the following:
Tetrahedral void and octahedral void
In close-packed structures, for every atom, it contains
The empty space between the shaded balls and hollow balls as shown in the diagram is called
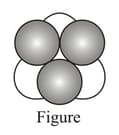
A double triangular void surrounded by three spheres above and three spheres below is called
How many numbers of tetrahedral voids are present in an unit cell:
The number of octahedral sites per sphere in a structure is:
A compound is formed by two elements and . The element forms and atoms of occupy rd of tetrahedral voids. What is sum of the ratio of atoms of & in the formula of the compound?
Which of the following statements is not true about the voids?
