Terminology in Thermodynamics
Terminology in Thermodynamics: Overview
This topic covers concepts, such as, Thermodynamics, Scope of Thermodynamics, Sign Conventions for Work & Factors Affecting Internal Energy etc.
Important Questions on Terminology in Thermodynamics
Which of the following are not state functions?
The work done during the expansion of a gas from a volume of against a constant external pressure of 3 atm is (1 L atm = 101.32 J):
For a cyclic process, which of the following is not true?
At constant temperature, for a given mass of an ideal gas
In thermodynamics, a process is called reversible when.
A reversible process is the one carried out in infinitesimal steps while, an irreversible process is carried out in a finite number of steps.
Differentiate between the reversible and irreversible process on the basis of entropy change.
The chemical energy is used to do _____ work when a fuel burns in an engine.
Write some energy transformations of chemical energy stored in different substance, molecules, atoms, etc.
In a hydrogen fuel-cell chemical energy is converted to
The thermodynamic terms surroundings, system and universe can be related as: The universe The system The surroundings.
Establish a relationship between the surroundings, system and universe.
Define the thermodynamic term surroundings.
Chemical energy cannot be directly used to draw water from a well.
Write an example for the conversion of chemical energy into kinetic energy.
How is potential energy of water is converted into electrical energy?
When a book is dropped, its _____ energy is transformed into kinetic energy.
Write an example for the conversion of chemical energy into electrical energy.
The diagram shows the pressure and volume relationship for one cycle of operation of an engine.
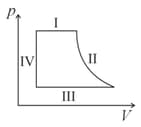
Which of the labelled parts of the cycle identify isobaric changes and adiabatic changes of state?
The process in which the value of is:
