Thermochemistry
IMPORTANT
Thermochemistry: Overview
This topic covers concepts such as Thermochemistry and Thermochemical Equations.
Important Questions on Thermochemistry
EASY
IMPORTANT
The branch of chemistry which deals with the study of heat exchange, entropy, enthalpy of a chemical reaction is called
HARD
IMPORTANT
Distribution of fraction of molecules with velocity is represented in the figure.
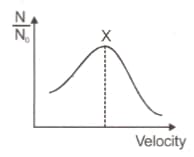
Velocity corresponding to point is
HARD
IMPORTANT
mole of a gas at bar and are compressed at constant temperature by use of a constant pressure of bar. How much work is done on the gas ?
HARD
IMPORTANT
The heat evolved in the following reaction to produce of water is
EASY
IMPORTANT
Standard enthalpies of formation of and are respectively. The order of their increasing stability will be
MEDIUM
IMPORTANT
How many grams of ice at can be melted by the addition of 500 J of heat? (The molar heat of fusion for ice is )
