Tests for Iron(III) Ion
Tests for Iron(III) Ion: Overview
This topic covers concepts, such as, Disodium Hydrogen Phosphate Solution Test for Iron(III) Ion, Potassium Ferricyanide Test for Iron(III) Ion, Sodium Acetate Solution Test for Iron(III) Ion and Ammonium Sulphide Solution for Iron(III) Ion etc.
Important Questions on Tests for Iron(III) Ion
Prolongated heating of ferrous ammonium sulphate is avoided to prevent
Consider following sequence of chemical reactions that have been carried out in laboratory.
(i) Unknown salt '' Distilled water
(ii) '' Colour of remains same (No reaction)
(iii) dil. Coloured solution
Then the unknown salt '' is:
An inorganic salt(S) reacts with solution to produce ppt. which is insoluble in dil. . Acidified green coloured aqueous solution of salt (S) is converted to yellow on long exposure of air, on bubbling in yellow solution elemental sulphur is produced.
Select CORRECT for given reactions.
On reaction of sodium hydroxide with Iron ion gives a pink coloured precipitate.
On reaction of sodium hydroxide with Iron ion gives a coloured precipitate. Identify the colour.
On adding sodium hydroxide drop by drop to a solution of ferrous sulphate, a dirty green precipitate is formed. Write the chemical formula of the compound formed of this colour.
(Enter your correct answer as )
Ammonium hydroxide reacts to distinguish solution from solution.
The colour of precipitate formed when sodium hydroxide is added to a solution of ferric chloride is _____.
A substance X is a yellow solution and gives a reddish-brown precipitate Y which remains insoluble in excess Ammonium hydroxide. Identify the substances X and Y and also write the complete balanced equation.
Match the following:
| (i)A solution of this compound gives a dirty green precipitate with sodium hydroxide | (a)Aluminum oxide |
| (ii)A metal which reacts with sodium hydroxide to evolve hydrogen gas. | (b)Ferrous sulphate |
| (iii)An amphoteric oxide which reacts with to give salt and water. | (c)Zinc |
How many of the given cations will give green colour in Borax bead test?
A pale green crystalline metallic salt dissolves freely in water and gives a brown precipitate on addition of aqueous The metallic salt solution also gives a black precipitate on bubbling in acidic medium. An aqueous solution of this decolourises the purple colour of the permanganate solution. The metal in the metallic salt solution is:
Consider the following statements,
S1 : Fe(OH)3 and Cr(OH)3 precipitates can be separated using NaOH + H2O2 but not by excess of NaOH alone.
S2 : Ag2CrO4 precipitate is soluble in dilute HNO3 and ammonia solution.
S3 : both HgI2 and BiI3 precipitates form colourless soluble complexes with excess of potassium iodide solution.
S4 : white precipitate of PbCl2 is turned black by H2S (not taken in excess) in saturated solution of KCl.
and arrange in the order of true / false.
Ferric ion forms a Prussian blue precipitate due to the formation of:
A compound is soluble in water. If ammonia is added, a red precipitate appears which is soluble in dilute . The compound has
A reagent which produces a dark blue precipitate with is :
Which of the following pairs of ions when mixed in dilute solutions may give precipitate ?
can be separated from by addition of
A light greenish coloured salt was soluble in water. On passing into the solution a black precipitate was obtained which dissolve readily in . The metal ion present is

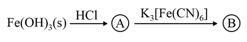
 is
is