Change of State
Change of State: Overview
This topic covers concepts, such as Change of State, Temperature Versus Time Graph for Heating of Ice, Mixing of Liquid and Vapour at Different Temperatures & Phase Diagram etc.
Important Questions on Change of State
Water and ice having equal masses are placed inside an insulated container. The initial temperatures of water and ice are and respectively. The equilibrium temperature of the system in degrees Celsius is close to (Note: take the specific heat of water ; latent heat of fusion )
The physical quantity that determines whether or not a given system is in thermal equilibrium with another system is called
The normal boiling point of water is. At
The normal boiling point of the liquid is approximately
How are vapour pressure and boiling point related
A substance is said to be a ________, if its melting point is above room temperature under atmospheric pressure.
At what temperature does water boil under normal conditions.
The normal boiling point of a liquid is the temperature at which it boils when the external pressure is ____________
Evaporation is the process of changing liquid into vapor,
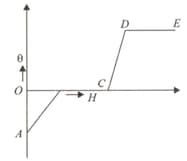
Heat given (H) to a substance was plotted against rise in temperature (0). Which of the following parts of the graph, most correctly
depicts the latent heat of the substance?
A thermos bottle containing coffee is vigorously shaken. If the coffee is considered a system, then the temperature of the coffee will
Which of the following is not an example of sublimable substances?
Which of the following is not an example of sublimable substances?
The melting point ( K) of aluminium is:-
Ice melts when energy in the form of ____ is supplied
The amount of heat required to convert a unit mass of solid into gas is
The normal boiling point of a liquid is that temperature at which vapour pressure of the liquid is equal to:
Two systems are in thermal equilibrium. The quantity which is common for them is
What is the triple point of water is
Liquid and solid states of a substance co-exist in thermal equilibrium during the process of: