Zeroth Law of Thermodynamics
Zeroth Law of Thermodynamics: Overview
This topic covers concepts, such as Thermodynamic System, System, Surroundings and Boundaries, Isolated System, Thermodynamic Equilibrium and Processes, Adiabatic Boundaries, Diathermic Boundaries & Zeroth Law of Thermodynamics etc.
Important Questions on Zeroth Law of Thermodynamics
A system is said to be an isolated system if
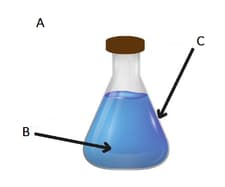
If we want to study the liquid inside the flask, we can define A, B and C as
What is a boundary for a thermodynamic system?
If a closed system has adiabatic boundaries, then atleast one boundary must be:
For an isolated system, the entropy:
Which of the following is constant in a diathermic boundary?
Which of the following is constant in a diathermic boundary?
Which one of the following is correct about an adiabatic boundary?
An open system is one in which
When the temperature of a body is equal to that of the surroundings then the body appears
A diathermic wall is one which
When the temperature of a body is equal to that of the surroundings then the body appears
Zeroth law of thermodynamics gives the concept of
Samples and are initially kept in the same state. They expand through adiabatic and isothermal processes respectively to the same final volume. If is final pressure of sample and that of sample . Then
What percentage of total heat supplied is used for doing work, If a gas is heated at constant pressure? (Given: for gas = )
Cotyledons are also called-
Equal molecules of two gases are in thermal equilibrium. If , and , are their respective pressures and volumes, then which of the following relation is true?
Human body is an example of
An adiabatic process occurs in
The human body is an example of
