Pressure - Volume Relationship
Pressure - Volume Relationship: Overview
This topic covers concepts such as Boyle's Law and Graphs Based on Boyle's Law.
Important Questions on Pressure - Volume Relationship
Boyle's law states that when the temperature of a gas is kept constant, the volume of a fixed mass of gas is inversely proportional to its pressure.
What type of graph is obtain when pressure is plotted against volume?
According to Boyle’s law, the volume of a gas is inversely proportional to its pressure at a constant temperature.
The plot of volume versus pressure at constant temperature is _____. (hyperbola/parabola)
When a large bubble rises from the bottom of a lake to the surface, its radius doubles. If atmospheric pressure is equal to that of a column of water of height . The depth of the lake is
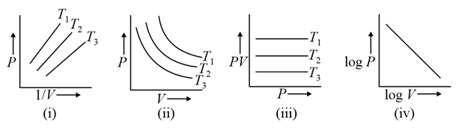
Graphs between pressure and volume are plotted at different temperatures. Which of the following isotherms represents Boyle's law as PV = constant?
A gas at the temperature is contained in a closed vessel. If the gas is heated through then percentage increase in its pressure will be
