Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.: Overview
This topic covers various concepts like Electron Dot Structure of Methane, , etc.
Important Questions on Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
For phenol, which of the following resonating structure is the most stable?
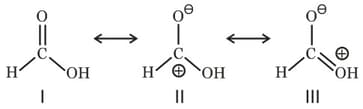
Among these canonical structures, the correct order of stability is
Which of the following statements are true.
(i) In the structure of the bond is shorter than the bond
(ii) All the bonds in are not equivalent.
(iii) is more reactive than
Based on lattice energy and other considerations which one of the following alkali metal chlorides is expected to have the highest melting point?
Based on lattice energy and other considerations which of the following alkali metal chloride is expected to have the highest melting point ?
a covalent solid.
Generally which is the larger charge in size one positive charge or one negative charge?
In acetylene molecule, sigma bond is formed by:
Amongst the following, the total number of species NOT having eight electrons around central atom in its outer most shell, is
The number of molecules from the following which contain only two lone pair of electrons is _____________
. Identify the correct order of standard enthalpy of formation of sodium halides.
The bond order and magnetic property of acetylide ion are same as that of
Consider the following statement
(A) molecules has a trigonal planar structure.
(B) Bond Length of is shorter than .
(C) Isoelectronic molecules or ions have identical bond order.
(D) Dipole moment of is higher than that of water molecule.
Choose the correct answer from the options given below:
How do cations and anions of an ionic compund exist in its solid state ?
What is a Sigma bond?
Draw the structure of tetra cyano ethylene.
is not a conductor of electricity in solid state whereas it does conduct electricity in aqueous solution as well as in molten state.
Calculate the number of lone pairs and bond pairs in molecule.
Write Lewis dot structure of carbon tetrachloride.
The number of electron pairs shared by the two carbon atoms which are bounded by a triple bond are:
