Specific Heat Capacity of Gases
Specific Heat Capacity of Gases: Overview
This topic deals with the concept of specific heat capacities of gases including monatomic, diatomic and polyatomic gases. With the aid of examples, it briefs on the formulas and their uses.
Important Questions on Specific Heat Capacity of Gases
Define molar specific heat capacity at constant volume and pressure.
The ratio of the molar heat capacities of a diatomic gas at constant pressure to that at constant volume is
A molecule of a gas has six degrees of freedom. Then, the molar specific heat of the gas at constant volume is
One gram mole of an ideal gas with the ratio of constant pressure and constant volume specific heats is mixed with gram moles of another ideal gas with . If the for the mixture is , then what will be the value of ?
A rigid triangular molecule consists degrees of freedom. The adiabatic constant of an ideal gas consisting of such molecules is
For the next three questions
An ideal diatomic gas is expanded so that the amount of heat transferred to the gas is equal to the decrease in its internal energy.
The molar specific heat of the gas in this process is given by C whose value is
Two mole of oxygen is mixed with eight mole of helium. The effective specific heat of the mixture at constant volume is _____.
Two moles of a certain gas at a temperature, are cooled isochorically so that the pressure of the gas gets reduced by times. Then, as a result of isobaric process, the gas is allowed to expand till its temperature gets back to the initial value. Find the total amount of heat absorbed by gas in this process.
Percentage change in volume of the solid, in the first is
Specific heat capacity of the liquid is
A calorimeter of Brass is of mass contains of water. of unknown material initially at is placed in calorimeter. Initial temperature of water is The final temperature of mixture is Then specific heat of unknown material is.
Specific heat of a solid does not depend on which of the following factors?
A thermally insulated piece of metal is heated under atmosphere by an electric current so that it receives electric energy at a constant power P. This leads to an increase of the absolute temperature T of the metal with time t as follows
Then the heat capacity is
Which one of the following would raise the temperature of of water at most, when mixed with [specific heat of water is ]
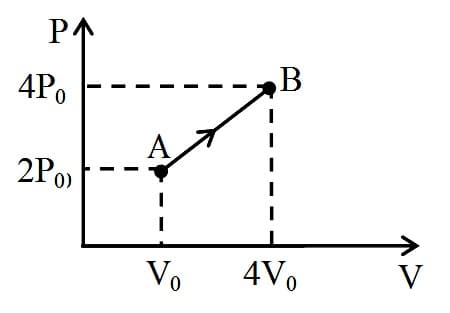
One mole of a monoatomic ideal gas undergoes the process, as in the given diagram. The specific heat for this process is
Assertion: The ratio of specific heat of gas at constant pressure and specific heat at constant volume is more for helium gas than for hydrogen gas.
Reason: Atomic mass of helium is more than that of hydrogen.
Assertion: The ratio for a diatomic gas is more than that for a monoatomic gas.
Reason: The molecules of a monoatomic gas have less degree of freedom than those of diatomic gas.
Assertion: The specific heat at constant pressure is greater than the specific heat at constant volume i.e., .
Reason: In case of specific heat at constant volume, the whole of heat supplied is used to raise the temperature of one mole of the gas through while in case of specific of heat at constant pressure, heat is to be supplied not only for heating 1 mole of gas through but also for doing work during expansion of the gas.
22 g of is mixed with 16 g of oxygen at 37. The temperature of the mixture is
The value of molar specific heat at constant pressure for one mole of triatomic gas (triangular arrangement) at temperature is the universal gas constant)
