Specific Heat
Specific Heat: Overview
This topic covers concepts, such as, Heat Capacity, Specific Heat Capacity, Variation of Specific Heat Capacity of Water with Temperature & Specific Heat of a Gaseous Mixture etc.
Important Questions on Specific Heat
The molar heat capacity of water at constant pressure is . When 1kJ of heat is supplied to 100g of water, which is free to expand, the increase in temperature of water is
During an adiabatic process, the pressure of a gas is found to be proportional to the cube of its absolute temperature. The ratio for the gas is . Find .
Two different liquids of same mass are kept in two identical vessel, which are placed in a freezer that extracts heat from them at the same rate causing each liquid to transform into a solid. The schematic figure below shows the temperaure vs time plot for the two materials. We denote the specific heat in the liquid states to be and for materials and respectively, and latent heats of fusion and respectively.
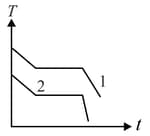
Choose the correct option.
Heat is supplied to a solid to raise its temperature across its melting point and boiling point Which of the following graphs correctly represents the relation between heat supplied and temperature ?
200 g water is heated from to . Ignoring the slight expansion of water, the change in its internal energy is close to (Given specific heat of water J/kg/K):
A current carrying wire heats a metal rod. The wire provides a constant power to the rod. The metal rod is enclosed in an insulated container. It is observed that the temperature in the metal rod changes with time as:
where is a constant with appropriate dimension while is a constant with dimension of temperature. The heat capacity of the metal is:
A person weighing takes in diet per day. If this energy were to be used in heating the body of person without any losses, then the rise in his temperature is (specific heat of human body )
Amongst object A and B, if the specific heat of object A is less than of object B, then
Calorie is the unit of which physical quantity?
Calorie is the unit of which physical quantity?
A cube of lead of at is supplied with a heat. Find the final temperature (in degree Celsius) of the lead cube. (Take, specific heat of lead is )
Calculate the heat energy (in joules) to raise the temperature of of water from to . (Take specific heat of water, )
The amount of heat required to raise the temperature of mass of water through is called its
Specific heat of a substance is given by , find out amount of heat required to raise the temperature of substance from to
The number of degrees of freedom of a gas whose specific heat capacity at constant pressure is is
(universal gas constant )
The ends of a uniform metal rod of length and area of cross-section are maintained at and . At the mid-point of the rod, heat is supplied at a constant rate of . If the temperature gradient on the higher temperature side of the rod in steady state is , then the value of is (Thermal conductivity of the metal )
A copper wire long is stretched by . If the energy stored in the stretched wire is converted to heat, calculate the rise in temperature of the wire. (Given: , Density of copper and Specific heat of copper
ice at is mixed with steam at . The minimum value of so that finally all ice and steam converts into water is,
(Use, , (melting) and (vaporization).)
Which statement is false for specific heat of water?
