Pressure of a Gas
Pressure of a Gas: Overview
This topic covers concepts, such as, Pressure inside Gas, Pressure Equation, Mean Speed of Molecules & Most Probable Speed of Molecules etc.
Important Questions on Pressure of a Gas
The ratio amongst most probable velocity, mean velocity and root mean square velocity is given by
The translational kinetic energy of molecule of a gas, at temperature is
One mole of an ideal gas is contained in a cube of volume ,as shown in the figure. One face is made up of absorbing material,
which absorb all the molecules falling on it.At any given time :
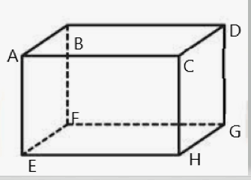
Time for successive collisions between walls ;-
(i) When there is collision between two walls then charge in momentum per second is .
Consider the following statements for air molecules in an airtight container: (I) The average speed of molecules is larger than root-mean-square speed. (II) The mean free path of molecules is larger than the mean distance between molecules. (III) The mean free path of molecules increases with temperature. (IV) The R.M.S. speed of nitrogen is smaller than oxygen molecules.Which of the above statements are correct?
Assertion: Consider a system of gas having N molecules; Instantaneous K.E. of few molecules can be greater than average K.E. of the molecules of the given gas.
Reason: Number of molecules having most probable speed is greater than number of molecules having average speed.
If the temperature of the gas is increased to three times, it's root mean square velocity becomes
Which of the following shows the correct relationship between the pressure and density of an ideal gas at constant temperature?
If the rms velocity of oxygen molecule at certain temperature is , the rms velocity for hydrogen molecule at the same temperature will be:
A cylinder of capacity is containing The total average translational kinetic energy of molecules is The pressure of in the cylinder will be (in )
The root-mean-square and most probable speed of the molecules in a gas are
Suppose a container is evacuated to leave just one molecule of gas in it. Let and represent the average speed and the rms speed of the gas.
The mean free path and RMS velocity of a nitrogen molecule at a temperature are and , respectively. The mean time between two successive collisions will be , what is the value of .
Assertion: Consider a system of gas having N molecules; Instantaneous K.E. of few molecules can be greater than average K.E. of the molecules of the given gas.
Reason: Number of molecules having most probable speed is greater than number of molecules having average speed.
At constant pressure, which of the following is true?
The mean free path of a gas sample is given by:
The gas having average speed four times as that of (molecular mass 64) at the same temperature, is
Let
Kinetic energy per unit volume is . The pressure exerted by the gas is given by
The rms speed of hydrogen is times the rms speed of nitrogen. If is the temperature of the gas, then
