Change of State
Change of State: Overview
This topic covers concepts such as Latent Heat, Latent Heat of Fusion, Latent Heat of Vaporisation, Latent Heat of Sublimation, Mixing of Liquid and Solid at Different Temperatures, Mixing of Liquid and Vapour at Different Temperatures, etc.
Important Questions on Change of State
Is latent heat released during sublimation?
At what temperature does water boil under normal conditions.
While ice is melted into water, its temperature increases.
Determine the amount of heat required to completely melt of ice (in ) given that latent heat of fusion for ice is .
Which of the following is not an example of sublimable substances?
Which of the following is not an example of sublimable substances?
Explain the process of sublimation.
The quantity of heat required to convert the unit mass of a substance from its liquid state to the gas state, at its boiling point without any change in its temperature is called the latent heat of fusion.
Observe the following temperature Vs. time graph and fill in the blank:
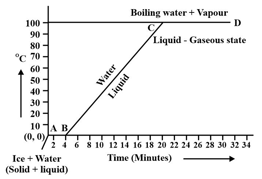
During transition of solid phase to liquid, the object absorbs _____ energy, but its temperature does not increase.
Temperature is a macroscopic concept.
A block of ice at is slowly heated and converted to steam at . Which of the following curves represent the phenomenon qualitatively?
Two systems are in thermal equilibrium. The quantity which is common for them is
Select the examples of regelation from the following.
The phenomenon in which the ice converts to liquid due to applied pressure and then re-converts to ice once the pressure is removed is called _____.
Latent heat depends on the mass of the substance.
The ice melts at a temperature lower than on application of pressure.
The phenomenon of regelation helps in skating.
The phenomenon in which the ice converts to liquid due to applied pressure and then re-converts to ice once the pressure is removed is called _____.
What is the critical temperature?
The value of latent heat of fusion of ice is .
