Heat Engines and Refrigerators
Heat Engines and Refrigerators: Overview
This topic covers concepts, such as, Carnot's Engine, Carnot Cycle, Heat Engine & Efficiency of Heat Engine etc.
Important Questions on Heat Engines and Refrigerators
An ideal Carnot engine working with source temperature and sink temperature , has efficiency . Then the value of the ratio is
A diatomic ideal gas is used in a carnot engine as the working substance. If during the adiabatic expansion part of the cycle, the volume of the gas increases from to , the efficiency of the engine is,
Even Carnot engine cannot give efficiency because we cannot
A Carnot engine works between constant temperature and of source and sink, respectively. For efficiency to be greatest
The efficiency of all reversible heat engines operating between the same heat reservoirs is :
A Carnot engine whose low-temperature reservoir is at has an efficiency of . It is desired to increase this to . If the temperature of the low-temperature reservoir remains constant, then the temperature of the high-temperature reservoir must be increased by how many degrees?
Consider a reversible engine of efficiency . When the temperature of the sink is reduced by , its efficiency gets doubled. The temperature of the source and sink respectively are
When temperture of heat reservoir increases by , the efficiency of the engine is nearly equal to __, if the temperatures and of heat reservoirs in an ideal Carnot engine are and respectively.
A refrigerator is to maintain the eatables, kept inside it, at . The coefficient of performance of the refrigerator, if the room temperature is , is
The efficiency of the Carnot engine is and the temperature of the sink is . If temperature of the source is kept constant and its efficiency is raised to , then the required temperature of the sink will be
The efficiency of the Carnot engine is and the temperature of the sink is . If the temperature of the source is kept constant and its efficiency raise to , then the required temperature of the sink will be
A reversible heat engine converts one- fourth of the heat input into work. When the temperature of the sink is reduced by its efficiency is doubled. The temperature in Kelvin of the source will be __________ .
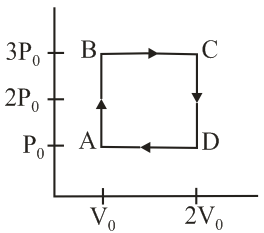
An engine operates by taking a monoatomic ideal gas through the cycle shown in the figure. The percentage efficiency of the engine is close to _____.
An ideal heat engine exhausting heat at is to have a efficiency. It must take the heat at:
An ideal refrigerator has a freezer at a temperature of . The coefficient of performance of the refrigerator is The temperature of the air (to which heat is rejected) will be:
The temperatures and of heat reservoirs in the ideal Carnot engine are and respectively. If increases by , the efficiency of the engine in percentage is
A Carnot engine absorbs of heat energy from a reservoir at and rejects of heat during each cycle, then the temperature of sink is
An ideal gas heat engine operates in a carnot cycle between and . It absorbs at the higher temperature. The amount of heat (in ) converted into work is equal to
A carnot refrigeration cycle absorbs heat at and rejects heat at If the cycle is absorbing at then work required per second is:
A heat engine is operating between to . If the engine absorbs heat, then which of the following is impossible amount of heat rejected by this heat engine.
