Heat Engines and Refrigerators
Heat Engines and Refrigerators: Overview
This topic covers concepts, such as, Carnot's Engine, Carnot Cycle, Heat Engine & Efficiency of Heat Engine etc.
Important Questions on Heat Engines and Refrigerators
A Carnot engine operates between a source and a sink. The efficiency of the engine is and the temperature of the is . If the efficiency is to be increased to then the temperature of the source must the increased by
The temperature of the sink of a Carnot engine is . In order to increase the efficiency of the Carnot engine from to , the temperature of the sink should be increased by
A diatomic ideal gas is used in a carnot engine as the working substance. If during the adiabatic expansion part of the cycle, the volume of the gas increases from to , the efficiency of the engine is,
Even Carnot engine cannot give efficiency because we cannot
A Carnot engine works between constant temperature and of source and sink, respectively. For efficiency to be greatest
The efficiency of all reversible heat engines operating between the same heat reservoirs is :
A Carnot engine whose efficiency is takes in heat from source maintained at a temperature of . It is desired to have an engine of efficiency . Then, the intake temperature for the same exhaust (sink) temperature must be
A Carnot engine having an efficiency as a heat engine, is used as a refrigerator. If the work done on the system is , the amount of energy absorbed from the reservoir at lower temperature is
Three designs are proposed for an engine which is to operate between and . Design A claims to produce of work per of heat input, design B of work per and design C of work per . Which of the designs would you choose?
Three Carnot engines operate in series between a heat source at temperature and heat sink at a temperature There are two other reservoirs at temperatures and The three engines are equally efficient if (given that
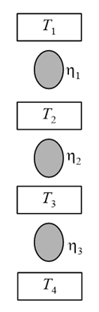
The efficiency of Carnot heat engine _____
calculate the work done on the gas in carnot cycle during compression.
Isothermal compression in Carnot cycle happened at _____.
The correct sequence of the processes taking place in a carnot cycle is
The third process is isothermal compression.
A Carnot engine whose low-temperature reservoir is at has an efficiency of . It is desired to increase this to . If the temperature of the low-temperature reservoir remains constant, then the temperature of the high-temperature reservoir must be increased by how many degrees?
Consider a reversible engine of efficiency . When the temperature of the sink is reduced by , its efficiency gets doubled. The temperature of the source and sink respectively are
When temperture of heat reservoir increases by , the efficiency of the engine is nearly equal to __, if the temperatures and of heat reservoirs in an ideal Carnot engine are and respectively.
A refrigerator is to maintain the eatables, kept inside it, at . The coefficient of performance of the refrigerator, if the room temperature is , is
The efficiency of the Carnot engine is and the temperature of the sink is . If temperature of the source is kept constant and its efficiency is raised to , then the required temperature of the sink will be
