Thermal Equilibrium
Thermal Equilibrium: Overview
This topic covers concepts, such as Thermodynamic Equilibrium and Processes, Adiabatic Boundaries and Diathermic Boundaries.
Important Questions on Thermal Equilibrium
If a closed system has adiabatic boundaries, then atleast one boundary must be:
Which of the following is constant in a diathermic boundary?
Which of the following is constant in a diathermic boundary?
Which one of the following is correct about an adiabatic boundary?
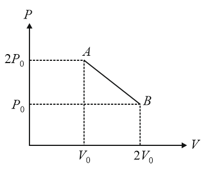
moles of an ideal gas undergoes a process as shown in the figure. The maximum temperature of the gas during the process will be:
When the temperature of a body is equal to that of the surroundings then the body appears
When the temperature of a body is equal to that of the surroundings then the body appears
Samples and are initially kept in the same state. They expand through adiabatic and isothermal processes respectively to the same final volume. If is final pressure of sample and that of sample . Then
What percentage of total heat supplied is used for doing work, If a gas is heated at constant pressure? (Given: for gas = )
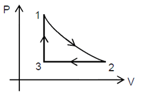
Which of the following is an equivalent V-T graph corresponding to the thermodynamic cycle given in the figure , where, is adiabatic. (Graphs are schematic and are not to scale)
Ice at is added to of water initially at in a vacuum flask. When of ice has been added and has all melted, the temperature of flask and contents is , When a further of ice is added and has all melted, the temperature of the whole becomes . Neglecting heat lost to surroundings the latent heat of fusion of ice is :
Equal molecules of two gases are in thermal equilibrium. If , and , are their respective pressures and volumes, then which of the following relation is true?
The thermo emf E (in volts) of a certain thermocouple is found to vary with Q (in C) according to equation is temperature of the hot function, the cold function being kept at . Then, the neutral temperature of the thermocouple is
There are four objects A, B, C and D. It is observed that A and B are in thermal equilibrium and C and D are also in thermal equilibrium. However, A and C are not in thermal equilibrium. We can conclude that
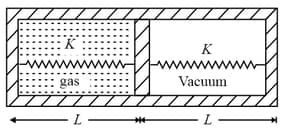
Area of piston is . When heat is supplied to the gas, it expands and displaces piston by , where, . Natural length of springs . Spring constant . The pressure of gas in final situation is (considering equilibrium)