Moseley’s Law
Moseley’s Law: Overview
This topic covers concepts, such as, Different Series in Characteristic X-rays, Wavelength and Energy of Emitted X-ray Photons, Identifying Elements from X-ray Spectrum, Bragg's Diffraction & Moseley's Law in Characteristic X-rays etc.
Important Questions on Moseley’s Law
The element which has a -rays line of wavelength is and
If (nical) is used as target material, then is minimum wavelength produced in -ray tube and is the wavelength of line. If the operating tube voltage is increased and (calsium) is used as target material in place of then the difference will :
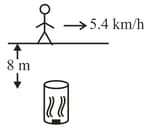
A student is jogging on a straight path with the speed per hour. Perpendicular to the path is kept a pipe with its opening from the road (see figure). Diameter of the pipe is At the other end of the pipe is a speaker emitting sound of towards the opening of the pipes. As the student passes in front of the pipe, she hears the speaker sound for seconds. is in the range (take speed of sound, ):
What is the atomic number of the element whose wavelength of line of characteristic $X-$ray is (Rydberg constant
The wavelength of a certain line in the -rays spectrum for tungsten is . What would be the wavelength of the same line for platinum The screening constant is unity.
A screen is place from a single slit, which is illuminated with light. If the distance between the first and third minima in the diffraction pattern is the width of the slit is
Use Moseley's law with to find the frequency of the -rays of . if the frequency of the -rays of is known to be
The wavelength of line from an element of atomic number is . From another element the wavelength of line is . What is the atomic number of second element?
A cobalt (atomic no. ) target is bombarded with electrons and the wave lengths of its characteristic -rays spectrum are measured. A second weak characteristic spectrum is also found due to an impurity in the target. The wavelength of the lines are (cobalt) and (impurity). Atomic number of the impurity is
If the radiation of has a wavelength array of calculate wavelength of the end array corresponding radiation of i.e., for assuming
The wavelength of line for an element of atomic number is Then what is the wavelength of line for an element of atomic number ?
The wavelength of the -rays produced by an -ray tube is . What will be the atomic number of anode material?
For characteristic -ray of some material, which of the following must be true?
The -rays arising from a cobalt target have a wavelength of Which of the following is true about the -rays arising from a nickel target ?
The -ray wavelength of line of platinum is known to be . What is the -ray wavelength of line of molybdenum ?
What should be the wavelength (in ) of X-ray used for getting the first order Bragg reflection at a glancing angle of for a crystal having a distance of between its two parallel planes? (Consider)
The existence of matter waves was conformed by G.P. Thomson by the phenomena:
In a Coolidge tube experiment for production of , electrons with energy are incident on the tungsten target . shell electrons of tungsten have energy. Choose the correct option regarding being emitted by the tube ?
The wavelength of electron beam is . When these waves are diffracted from a metal foil having . At angle first maxima will be present, then what will be the value of :
A small aperture is illuminated with parallel beam of light having wavelength . If the first minima is formed at an angular position of then the size of aperture is
