Einstein's Equation of Photoelectric Effect
Einstein's Equation of Photoelectric Effect: Overview
This topic covers concepts, such as Quanta of Energy of Radiation, Maximum Kinetic Energy of Photoelectrons, Range of Kinetic Energy of Photoelectrons, Einstein's Equation of Photoelectric Effect, Explanation of Einstein's Photon Theory, etc.
Important Questions on Einstein's Equation of Photoelectric Effect
The maximum kinetic energy of a photoelectron is . Its stopping potential is
The Einstein’s photoelectric equation is:
The Einstein’s photoelectric equation is:
The following graph shows the variation of stopping potential with frequency of the incident radiation for two photosensitive metals and .
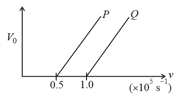
Which metal has
(i) a smaller threshold wavelength?
(ii) smaller kinetic energy?
The following graph shows the variation of stopping potential with the frequency of the incident radiation for two photosensitive metals and :
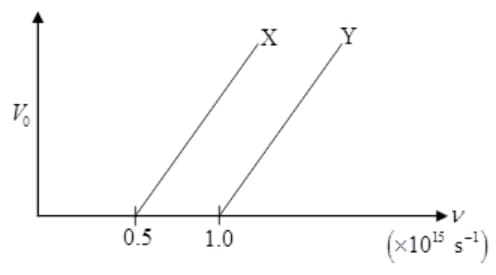
(i) Which of the metals has larger threshold wavelength? Give reason.
(ii) Explain, giving reason, which metal gives out electrons, having larger kinetic energy, for the same wavelength of the incident radiation.
The graph between frequency of incident radiations and stopping potential for a given photosensitive material is as follows.
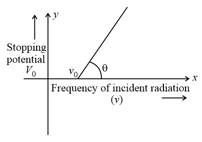
What information can be obtained from the value of the intercept on the potential axis?
Show graphically how the stopping potential for a given photosensitive surface varies with the frequency of the incident radiation.
In the photo-electron emission, the energy of the emitted electron is
The maximum kinetic energy of electrons emitted in the photoelectric effect is linearly dependent on the________________ of the incident radiation.
Einstein's theory of photoelectric effect establishes
In another experiment, a source of constant intensity and variable frequency is incident on a metallic surface. The graph shows the variation of the stopping potential with photon frequency , for a particular value of intensity.
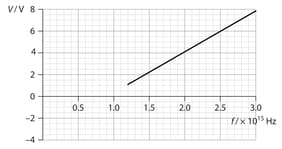
Use the graph to estimate the longest wavelength of light that will result in electron being emitted from the surface.
The threshold wavelength for ejection of photoelectrons from a metal is . The stopping voltage for the photoelectrons emitted from the surface of the metal when illuminated with light of wavelength is
Planks constant
Light of wave length is incident on Barium. Photo electrons emitted from the surface of barium describe a circle of radius in a magnetic field of intensity . Then, what is the work function of the Barium? (Given, for electron, )
In a photoelectric experiment, increasing the intensity of incident light:
In a photoelectric experiment, ultraviolet light of wavelength is used with lithium cathode having work function . If the wavelength of incident light is switched to , find out the change in the stopping potential.
In a photoelectric effect experiment, the slope of the graph between the stopping potential and the incident frequency will be
Radiation of wavelength is incident on a photocell. The fastest emitted photoelectron has a speed . If the wavelength is changed to , the speed of the fastest emitted photoelectron will be
When photons of wavelength are incident on an isolated sphere, the corresponding stopping potential is found to be V. When photons of wavelength are used, the corresponding stopping potential was thrice that of the above value. If light of wavelength is used then find the stopping potential for this case:
Ultraviolet radiation of 6.2 eV falls on an aluminium surface (work function 4.2 eV). The kinetic energy of the fastest electron emitted is approximately
An ultraviolet light of wavelength irradiates a photocell made of molybdenum metal. If the stopping potential is 1.5 V, what is the work function of the metal ?
(Planck's constant = 6.6 × 10–34 J s)
