Photoelectric Effect and Wave Theory of Light
Photoelectric Effect and Wave Theory of Light: Overview
This topic covers the concept of Photoelectric Effect and Wave Theory of Light.
Important Questions on Photoelectric Effect and Wave Theory of Light
The typical electric field required for electrons to be emitted from surfaces of metals by the "field emission" process is about
Calculate the initial voltage applied to an -ray tube, if the voltage is increased to times and the minimum wavelength of an -ray continuous spectrum is shifted by .
For a certain metal if the incident frequency v is five times the threshold frequency , then the maximum velocity of the photoelectrons is . What will be the maximum velocity of photoelectrons if ?
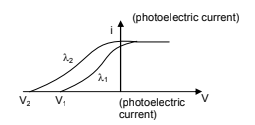
For a photoelectric effect setup, in the following diagram if then
A photoelectron ejected from a metal surface of work function by a photon of energy enters into a uniform magnetic field of induction in a direction perpendicular to the field and describes a circular path of radius which is given by (Symbols have the usual meaning)
Huygen's wave theory of light could not explain
If light of wavelength is falling on a sensitive surface and the total energy received by the surface is , then the number of photons falling on the surface will be,
The value of X-intercept is-
What is the of photo electrons
The maximum kinetic energy of electrons in a photoelectric effect depends on
A photocell employs photoelectric effect to convert
The photoelectric effect represent that ...........
In photoelectric effect, if the energy required to overcome the attractive forces on the electron, (work functions) of Li, Na and Rb are 2.41 eV, 2.30 eV and 2.09 eV respectively, the work function of 'K' could approximately be in eV
When ultraviolet radiation is incident on a surface, no photoelectrons are emitted. If a second beam causes photoelectrons to be ejected, it may consist of
Which is/are incorrect statements regarding photoelectric effect?
A photodetector used to detect the wavelength of 1700 nm, has energy gap of about
Photoelectric effect can be observed if a photon collides with a
Photoelectric effect supports quantum nature of light because
1. There is minimum frequency of light below which no photoelectrons are emitted.
2. Electric charge of photoelectrons is quantized.
3. Maximum kinetic energy of photoelectrons depends only on the frequency of light and not on its intensity.
4. Even when metal surface is faintly illuminated the photoelectrons leave the surface immediately.
There are photons of frequency in a beam of light. In an equally energetic beam there are photons of frequency . Then the correct relation
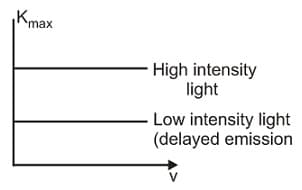
Statement I : According to classical physics, the plot between maximum kinetic energy of the ejected photoelectrons versus frequency in photoelectric experiments would be as shown.
Statement II : From classical theory, the photoelectric effect cannot be explained.