Atomic Models Rutherford’s Experiment
Atomic Models Rutherford’s Experiment: Overview
This topic covers concepts, such as, Atomic Model, Brief History of Atomic Structure Upto Bohr Model, Total Energy of Electron in Hydrogen Atom Using Rutherford Model of Atom & Distance of Closest Approach in Alpha Particle Scattering Experiments etc.
Important Questions on Atomic Models Rutherford’s Experiment
In Rutherford scattering experiment, the correct angle of scattering of α− particles for impact parameter equal to zero is
In Rutherford's experiment, the alpha particles that come closer to the nuclei are
Out of the following statements regarding Rutherford's model, which of the following is/are correct?
a. Most of the space inside an atom is empty.
b. The electrons revolve around the nucleus under the influence of coulomb force acting on them.
c. Most part of the mass of the atom and its positive charge are concentrated at its centre.
d. The stability of atom was established by the model.
Velocity of electron orbiting around nucleus of hydrogen atom is proportional to radius (r)
Radius of the smallest orbit of hydrogen atom is
In an atom , find the number of neutrons.
In an atom , find the number of protons.
In an atom , find the number of electrons.
The gravitational force between a -atom and another particle of mass will be given by Newton's law , where is in metre and
Geiger-Marsden experiment is related with the size of the-
A proton of mass and charge is projected from a very large distance towards an -particle with velocity Initially -particle is at rest, but it is free to move. If gravity is neglected, then the minimum separation along the straight line of their motion will be
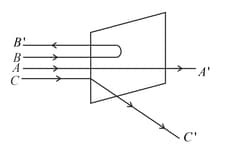
A beam of fast moving alpha particles were directed towards a thin film of Gold. The parts and of the transmitted and reflected beams corresponding to the incident parts and of the beam are shown in the adjoing diagram. The number of alpha particles in
- particle consists of
In Rutherford's experiment, an -particle of mass and charge moves at high speed , along the -axis. It is initially near , and it ends up near The gold nucleus having charge is fixed at the point . As the -particle passes the stationary nucleus, its -component of velocity does not change appreciably, but it acquires a small velocity in the -direction. The angle through which the -particle is deflected is,
The total energy of an electron in the hydrogen atom is . The kinetic energy of the electron will be:
On which of the following factors does the trajectory of an alpha particle depend?
The significant result deduced from the Rutherford's scattering experiment is that
A standing wave in a string 35 cm long has a total of six nodes including those at the ends. Hence, wavelength of the standing wave is
Rutherford -scattering demonstrates the existence of which of the following ?
What is the conclusion of Rutherford’s alpha particle scattering experiment ?
