Fission and Fusion Processes
Fission and Fusion Processes: Overview
This topic covers concepts, such as Nuclear Fission, Nuclear Fusion, Atom Bomb, Nuclear Chain Reaction, Critical Size and Critical Mass for an Atom Bomb, Hydrogen Bomb, Application of Nuclear Fission, etc.
Important Questions on Fission and Fusion Processes
If the nucleons of a nucleus are separated far apart from each other, the sum of masses of all these nucleons is larger than the mass of the nucleus.
Calculate the energy released if nucleus emits an particle.
Given:
Atomic mass of
Atomic mass of
Atomic mass of alpha-particle
How many number of neutrons are released in a fission of U 235?
How do you calculate fission rate?
What percentage of fission is high?
What is the mass of the product of nuclear fission reaction compared to the mass of the original product?
Identify nuclear fusion reaction
Identify nuclear fusion reaction.
If of energy is released in the fission of one nucleus of , the number of nuclei that must undergo fission to release an energy of is
If energy is released in the fission of a single nucleus, the number of fissions required per second to produce kilowatt power shall be (Given
What is the power output of reactor if it takesto use up of fuel, and if each fission gives of usable energy?
For a deuteron and an alpha particle, binding energies per nucleon are , and respectively then the energy released in the fusion reaction is :
Identify which of the following is a fusion reaction.
In the following reaction, find the value of ''.
The number of ideal diffusion steps that would be required to produce pure is
A heavy nucleus breaks into two nuclie and . Energy released during fission reaction is:
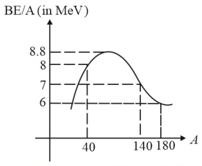
In the fusion reaction the masses of deuteron, helium and neutron expressed in are and respectively. If of deuterium undergoes complete fusion, find the amount of total energy released.
Which of the following is fusion process?
Assuming that about of energy is released per fusion reaction then the mass of consumed per day in a fusion reactor of power will approximately be
The energy released in the explosion of an atom bomb is mainly due to ................
