Mass Defect and Binding Energy
Mass Defect and Binding Energy: Overview
This topic covers concepts, such as Mass Defect, Binding Energy, Binding Energy per Nucleon, Dependence of Nuclear Stability on Binding Energy per Nucleon, Binding Energy per Nucleon versus Mass Number Graph, Mass Spectrometer, etc.
Important Questions on Mass Defect and Binding Energy
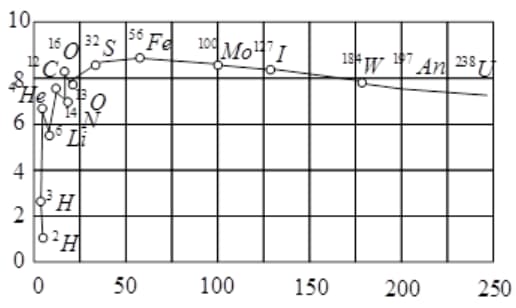
The plot of the binding energy per nucleon versus the mass number A for a large number of nuclei, is shown in Fig. In which range the binding energy per nucleon is constant?
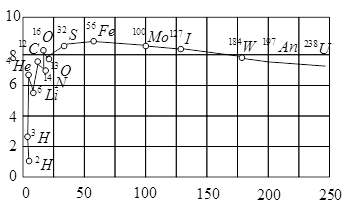
The plot of the binding energy per nucleon versus the mass number A for a large number of nuclei, is shown in Fig. In which range the binding energy per nucleon is constant?
Calculate the energy released in MeV in the following nuclear reaction :<
[Mass of = 238.05079 u]
Mass of = 234.043630 u
Mass of = 4.002600 u
1 u = 931.5 MeV
Calculate the binding energy per nucleon of nucleus.
Given:
Mass of ,
Mass of proton ,
and
In the nuclear process, stands for _______
Two deuterons undergo fusion to form a helium nucleus as . Given binding energy per nucleon for and are and , respectively. The -value is
The difference between the mass of a nucleus and the sum of the masses of the individual nucleons is Which of the following is approximately the binding energy of the nucleus?
Binding energy of a nucleus is,
The binding energy per nucleon is maximum in the case of,
The anti-particle of electron is known as
The binding energy per nucleon is maximum in the case of
If a proton and anti-proton come close to each other and annihilate, how much energy will be released?
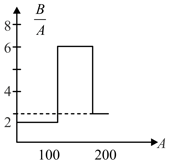
Assume that the nuclear binding energy per nuclear versus mass number as shown in the figure. Use this plot to choose the correct choice (s) given below.
The questions given below are based on the following paragraph. A nucleus of mass is at rest and decays into two daughter nuclei of each. Speed of light is c.
The binding energy per nucleon for the parent nucleus is and that for the daughter nuclei is then
A -ray photon creates an electron, positron pair. The rest mass of electron is 0.5 MeV. KE of the electron - positron pair system is 0.78 MeV. Then the energy of -ray photon is
In nuclear fusion, One gram hydrogen is converted into 0.993gm.If the efficiency of the generator be 5%,then the energy obtained in KWH is
Assume that a neutron breaks into a proton and electron. The energy released during this process is (Mass of neutron , mass of electron ) ,
The binding energy per each nucleon in the neighborhood of medium nuclei is 8.5 MeV and the binding energy per each nucleon is about 7.6 MeV in the neighborhood of Uranium. The energy released in the fission of is
The binding energy per nucleon of is and that of is . The energy required to remove one neutron from is
The atomic mass of is and that of is . The minimum energy required to remove the least tightly bound proton is (mass of a proton is )
