Composition and Size of Nucleus
Composition and Size of Nucleus: Overview
This topic covers concepts such as composition and structure of nucleus, nucleons, nuclear density, nuclear mass, and equivalence of mass and energy.
Important Questions on Composition and Size of Nucleus
Two nuclei have mass numbers in the ratio . The ratio of their nuclear radii would be:
Two nuclei have mass numbers in the ratio . The ratio of their nuclear radii is:
Assuming the nuclei to be spherical in shape how does the surface area of a nucleus of mass number compare with that of a nucleus of mass number ?
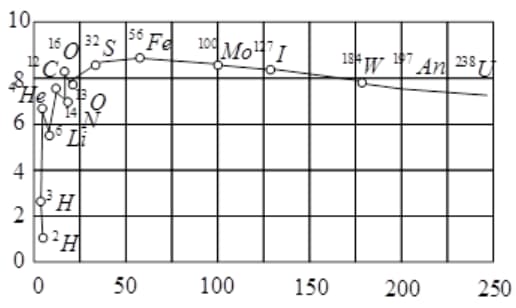
The plot of the binding energy per nucleon versus the mass number A for a large number of nuclei, is shown in Fig. In which range the binding energy per nucleon is constant?
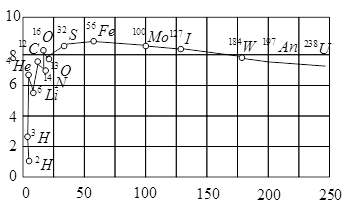
The plot of the binding energy per nucleon versus the mass number A for a large number of nuclei, is shown in Fig. In which range the binding energy per nucleon is constant?
Calculate the energy released in MeV in the following nuclear reaction :<
[Mass of = 238.05079 u]
Mass of = 234.043630 u
Mass of = 4.002600 u
1 u = 931.5 MeV
Calculate the binding energy per nucleon of nucleus.
Given:
Mass of ,
Mass of proton ,
and
The value of binding energy per nucleon of nucleus is
Given:
Mass of nucleus
Mass of proton
Mass of neutron
and
A neutron is absorbed by a nucleus with the subsequent emission of an alpha particle.
Calculate the energy released, in , in this reaction.
[Given: mass mass (neutron)
Mass (alpha particle) and
Mass (tritium)
[ Take ]
The binding energies per nucleon for deuteron () and helium () are and respectively. The energy released when two deuterons fuse to form a helium nucleus () is
In process of nuclear fission of , the mass lost is . The efficiency of power house's fission reactor is . To obtainmega watt power from the power house how much uranium required per hour
Convert mass into megaelectron volt.
A nuclear fusion reaction is given by. If atomic mass of He is and that of is amu then the Q-value of the reaction will be approximately
Given below are two statements: one is labelled as Assertion A and the other is labelled as Reason R
Assertion A : The binding energy per nucleon is practically independent of the atomic number for nuclei of mass number in the range to .
Reason R : Nuclear force is short ranged.
In the light of the above statements, choose the correct answer from the options given below
A nucleus with mass number and binding energy per nucleon as breaks into two fragment each with mass number . If each fragment nucleus has binding energy per nucleon as the total gain in binding energy is .
For a nucleus having mass number A and atomic number
A. The surface energy per nucleon
B. The Coulomb contribution to the binding energy .
C. The volume energy
D. Decrease in the binding energy is proportional to surface area.
E. While estimating the surface energy, it is assumed that each nucleon interacts with 12 nucleons. ( and are constants)
Choose the most appropriate answer from the options given below:
For the given radioactive decay , binding energy per nucleon of and are and . The is equal to
Given below are two statements : one is labelled as Assertion A and the other is labelled as Reason R
Assertion A : The nuclear density of nuclides can be arranged as
Reason R : The radius of nucleus is related to its mass number as , where is a constant.
In the light of the above statement, choose the correct answer from the options given below :
Find the energy equivalent of one atomic mass unit, first in Joule and then in MeV. Using this, express the mass defect of in .
Binding energy of a Nitrogen nucleus , given
