Lewis Symbols (or) Lewis Dot Structures
Important Questions on Lewis Symbols (or) Lewis Dot Structures
Represent the following atoms using Lewis notation:
Lithium
Represent the following atoms using Lewis notation:
Calcium
Represent the following atoms using Lewis notation:
Beryllium
Represent the molecule by using Lewis notation.
Draw a simple diagram to show how electrons are arranged in the following covalent molecule.
Chlorine
Draw a simple diagram to show how electrons are arranged in the following covalent molecule.
Water
Draw a simple diagram to show how electrons are arranged in the following molecule:
Calcium oxide
A chemical compound has the following Lewis notation:
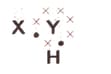
Suggest a name for the elements and .
A chemical compound has the following Lewis notation:
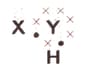
How many covalent bonds are there in the molecule?
How Lewis dot structure helps in understanding bond formation between atoms?
A chemical compound has the following Lewis notation:
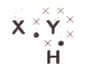
What is the valency of element ?
Two chemical reactions are described below.
(i) Nitrogen and hydrogen react to form ammonia .
(ii) Carbon and hydrogen bond to form a molecule of methane .
For each reaction give; the Lewis structure of the product that is formed.
In bromine gas , calcium chloride and carbon dioxide which contain a double bond?
Represent the molecule of carbon dioxide using Lewis notations.
A chemical compound has the following Lewis notation:
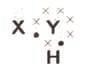
What is the valency of element ?
Represent the molecule of calcium chloride using Lewis notations.
Represent the molecule of bromine gas using Lewis notation.
A chemical compound has the following Lewis notation:
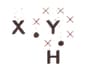
How many valence electrons does element have?

