Specific Heat Capacity
Specific Heat Capacity: Overview
This topic deals with the heat capacity of any substance. It explains specific and molar heat capacity at constant temperature and pressure with derived formulas. The values of specific and molar heat capacities of materials also given here.
Important Questions on Specific Heat Capacity
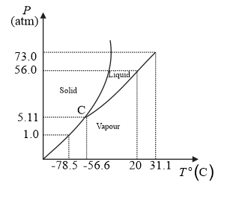
Answer the following questions based on the P–T phase diagram of
is heated to a temperature and compressed isothermally. What changes in its properties do you expect to observe?
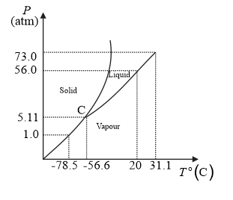
Answer the following questions based on the P–T phase diagram of
What happens when at pressure is cooled from room temperature at constant pressure?
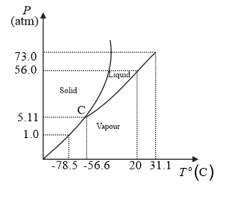
Answer the following questions based on the P–T phase diagram of :
at 1 atm pressure and temperature is compressed isothermally. Does it go through a liquid phase?
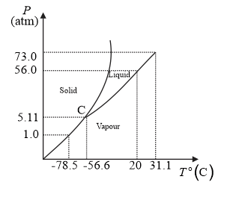
The following questions based on the phase diagram of carbon dioxide:
At what temperature and pressure can the solid, liquid and vapour phases of co-exist in equilibrium?
A drilling machine is used to drill a bore in a small aluminium block of mass . How much is the rise in temperature of the block in minutes, assuming of power is used up in heating the machine itself or lost to the surroundings? Specific heat of aluminium .
