Thermionic Emission and Cathode Ray Tube
Important Questions on Thermionic Emission and Cathode Ray Tube
Figure shows a radioactive source in a thick lead walled container having a narrow opening. The radiations pass through an electric field between the plates and
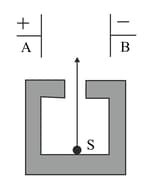
Why is the source kept in a thick lead walled container with a narrow opening ?
Figure shows a radioactive source in a thick lead walled container having a narrow opening. The radiations pass through an electric field between the plates and
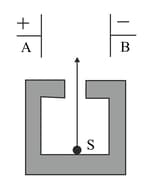
Complete the diagram to show the paths of and radiations.
The diagram in Figure shows a radioactive source placed in a thick lead walled container. The radiations given out are allowed to pass through a magnetic field. The magnetic field (shown as ) acts perpendicular to the plane of paper inwards. Arrows show the paths of the radiations and

Explain clearly how you used the diagram to arrive at the answer.
The diagram in Figure shows a radioactive source placed in a thick lead walled container. The radiations given out are allowed to pass through a magnetic field. The magnetic field (shown as ) acts perpendicular to the plane of paper inwards. Arrows show the paths of the radiations and

Name the radiations labelled and
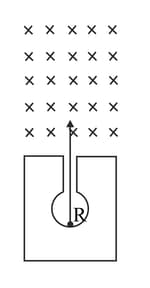
Figure shows a mixed source of alpha and beta particles in a thick lead walled container. The particles pass through a magnetic field in a direction perpendicular to the plane of paper inwards as shown by (a) Show in the diagram how the particles get affected.
[Hint : Alpha particles will deflect to the left while beta particles to the right]
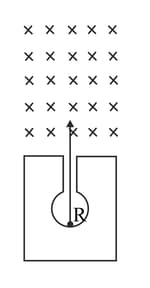
Figure shows a mixed source of alpha and beta particles in a thick lead walled container. The particles pass through a magnetic field in a direction perpendicular to the plane of paper inwards as shown by (a) Show in the diagram how the particles get affected. (b) Name the law used in part (a).
[Hint : Alpha particles will deflect to the left while beta particles to the right]
A certain nucleus (mass number and atomic number ) is radioactive and becomes a nucleus (mass number and atomic number ) by the loss of a particle.
Explain how you arrived at your answer.
What is the mass number of the nucleus ?
A certain nucleus (mass number and atomic number ) is radioactive and becomes a nucleus (mass number and atomic number ) by the loss of a particle.
State the change in the form of a reaction.
Does the composition of nucleus change if it emits a -radiation ?
A certain nucleus (mass number and atomic number ) is radioactive and becomes a nucleus (mass number and atomic number ) by the loss of a particle.
What particle was emitted ?
The nucleus emits a -particle and is transformed into a nucleus What is the composition of ?
An atomic nucleus is composed of protons and neutrons. The nucleus emits an -particle and is transformed into a nucleus . What is the composition of ?
An element disintegrates by -emission and the new element suffers two further disintegrations, both by -emission, to form an element . Explain the fact that and are the isotopes.
A nucleus of stable phosphorus has protons and neutrons.
What will be the atomic number and mass number of new nucleus formed by decay of a -particle by the radio phosphorus in part (b) ?
A nucleus of stable phosphorus has protons and neutrons.
The nucleus of radio phosphorus has one neutron more than the stable nucleus. What will be its atomic number and mass number ?
A nucleus of stable phosphorus has protons and neutrons.
What is its atomic number and mass number.
A nucleus is -radioactive.
What general name is given to the product nucleus with respect to
A nucleus is -radioactive.
Write the equation representing -decay.
A nucleus is -radioactive.
What are the numbers and called ?

