Acids
Acids: Overview
This topic introduces the acids with its classification into organic and inorganic acids. It discusses the role of water in the dissociation of an acid and uses of mineral acids in industry. It also explains the chemical nature of acids.
Important Questions on Acids
Which statement about the following equilibrium is true?
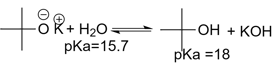
is present in the gastric juice of our body then how it becomes inorganic acid?
Which of the following does not belong to strong acid?
Which one of the following is the weakest acid?
One of the following is not an organic acid.This is
Name the element which is common to all acids?
- X is a strong acid with a pH value of 2.
- Y is a base that ionizes nearly 100% in water.
- Z is a base that can release hydroxide (OH) ions when dissolved in water. According to the information given above, in which of the following are the items X, Y, and Z correctly given?
Which of the following organic acid is present in tomato?
Mineral acids are stronger acids than carboxylic acids because
Mineral acids are completely ionised
Carboxylic acids are completely ionised
Mineral acids are partially ionised
Carboxylic acids are partially ionised
In the following reactions which property of an underlined substance is wrongly stated?
Which of the following ionisation equation for acid or base is wrong?
Equal volume aqueous solutions prepared with equal number of moles of KOH, HCl, KBr and HNO3 are mixed in a single container.The resulting solution obtained
The aqueous solution of which of the following is not acidic?
Item X shows following properties
- Its aqueous solution allows the passage of electric current.
- The number of moles of H+ ions in the aqueous solution is greater than the number of moles of OH- ions.
Which of the following cannot be an item of X?
