Preparation of Salts
Preparation of Salts: Overview
This topic teaches us various methods of preparation of salts and the reactions involved in their preparation. It also explains the titration method that is used in the laboratories to prepare salt.
Important Questions on Preparation of Salts
Milk of Magnesia is related with whose?
In the given reaction cycle
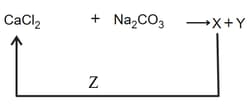
and respectively are
Monosodium glutamate is commonly known as
When hard water is evaporated completely, the white solid remains in the container. It may be due to the presence of _____.
. Carbonates of Ca and Mg
. Sulfates of Ca and Mg
. Chlorides of Ca and Mg
Select the answer using the code given below:
The nature of aqueous solution of is
Which of the following elements’ salts are most soluble?
White crystalline powder on reaction with phenolphthalein gives
Which of the following gas is liberated when zinc is added to dilute sulphuric acid?
What happens when dilute sulphuric acid is poured on zinc granules?
Reaction of copper hydroxide and dilute produces _____ .
Which of the following compound is formed when copper oxide which react with water or dilute is _____ .
What happens when copper oxide reacts with dilute sulphuric acid?
Which chemical reaction correctly represents the reaction between iron and dilute ?
What happens when iron reacts with dilute sulphuric acid?
Which of the following gases turn water milky?
Which of the following ions present in low concentration in drinking water is essential for the normal growth of teeth but harmful to teeth at high concentration?
When the same amount of zinc is treated separately with excess of sulphuric acid and excess of sodium hydroxide, the ratio of volumes of hydrogen evolved is -
