Thomson's model of the atom
Thomson's model of the atom: Overview
In this topic, we will understand Thomson’s model of an atom with the diagrammatic representation. We will know about the experiment done by Thomson on watermelon. It also explains the limitations of Thomson’s model.
Important Questions on Thomson's model of the atom
Metal of which foil was used in Rutherford experiment?
When -rays strike a thin gold foil then
(i) Atoms are neutral electrically.
(ii) Atomic diameter is approximately cm.
(iii) Atom is a positively charged sphere and electrons are distributed within it.
Which of the above views in Joseph Thomson's model of the atom are no longer valid today?
Atomic models have been improved over the years. Arrange the following atomic models in their chronological order.
(i) Rutherford's atomic model.
(ii) Thomson's atomic model
(iii) Bohr's atomic model
In Thomson's model of an atom, which of the following statement are correct?
(1) The mass of the atom is assumed to be uniformly distributed over the atom.
(2) The positive charge is assumed to be uniformly distributed over the atom.
(3) The electrons are uniformly distributed in the positively charged sphere.
(4) The electrons attract each other to stabilize the atom.
The first model of an atom was given by
Nucleus was discovered by Rutherford in the alpha particle scattering experiment.
Rutherford's alpha particle scattering experiment established that:
Rutherford's alpha Scattering experiment showed that:
The first model of an atom is given by
Who proposed ‘plum pudding’ model of an atom?
Match the following:
| 1. Thomson's atomic model | a. Negatively charged |
| 2. Rutherford's model | b. Neutrons and protons |
| 3. Electron | c. Positively charged |
| 4. Anode rays | d. Plum pudding model |
| 5. Nucleons | e. Planetary model of an atom |
The Plum Pudding Model of an atom was given by:
Name the scientist who proved the existence of electron. (Ernest Rutherford / J. J. Thomson / James Chadwick)
Match the column I with column II and select the correct answer by choosing an appropriate option.
| Column I | Column II |
| P. Neutron | 1. Rutherford's atomic model |
| Q. Plum pudding model | 2. Bohr's atomic model |
| R. Mass of the atom is concentrated at the centre of the atom | 3. Thomson's atomic model |
| S. Stationary orbit | 4. Chadwick |
Which model of an atom is depicted by the given figure?
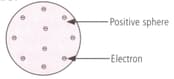
_____ was the first one to propose a model for the structure of an atom.
Describe Thomson's model of the atom. Which subatomic particle was not present in Thomson's model of the atom?
Why is an atom neutral inspite of the presence of charged particles in it?
