Atom
Atom: Overview
This topic covers concepts, such as, Naming of Elements, Size of Atom,Atomic Mass and Atomic Masses of Some Elements etc.
Important Questions on Atom
There are four different metal halides-A, B, C and D. The ratio of the number of constituent atoms (i.e., metal and halogen atoms present) in one gram molecule of those metal halides are respectively. Find the valency of the metals present in those metal halides. Give reasons in support of your answer.
One molecule of calcium phosphate reacts with silica and coke and gives rise to calcium silicate, phosphorus and carbon monoxide. Calculate the mass of carbon monoxide obtained.
The table lists the symbols for some common elements
| Sodium | Uranium | Nitrogen | Zinc | Chlorine | |
|
Symbol of the elementnce 1 |
Choose the correct statement for the symbols of different elements?
Atoms cannot been seen with eyes then, how can we say the exact number of electron in an atom?
Why is some of the name of atom are given in Latin language?
What is the difference between atoms and particles
Cations are electrically neutral particles which may or may not exist independently in a solution.
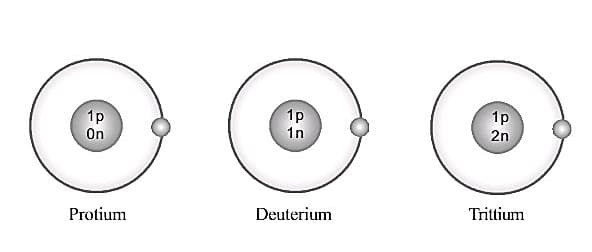
Among these atoms which is the particle that differs in number ?
Write the symbols of following elements: Beryllium, Carbon, Oxygen, Sodium, Silicon, Argon.
Explain why is taken as a reference to compare the atomic mass of element?
Which of the following is the SI unit of atomic mass unit?
Which of the following is taken as a reference to compare the atomic mass of element?
